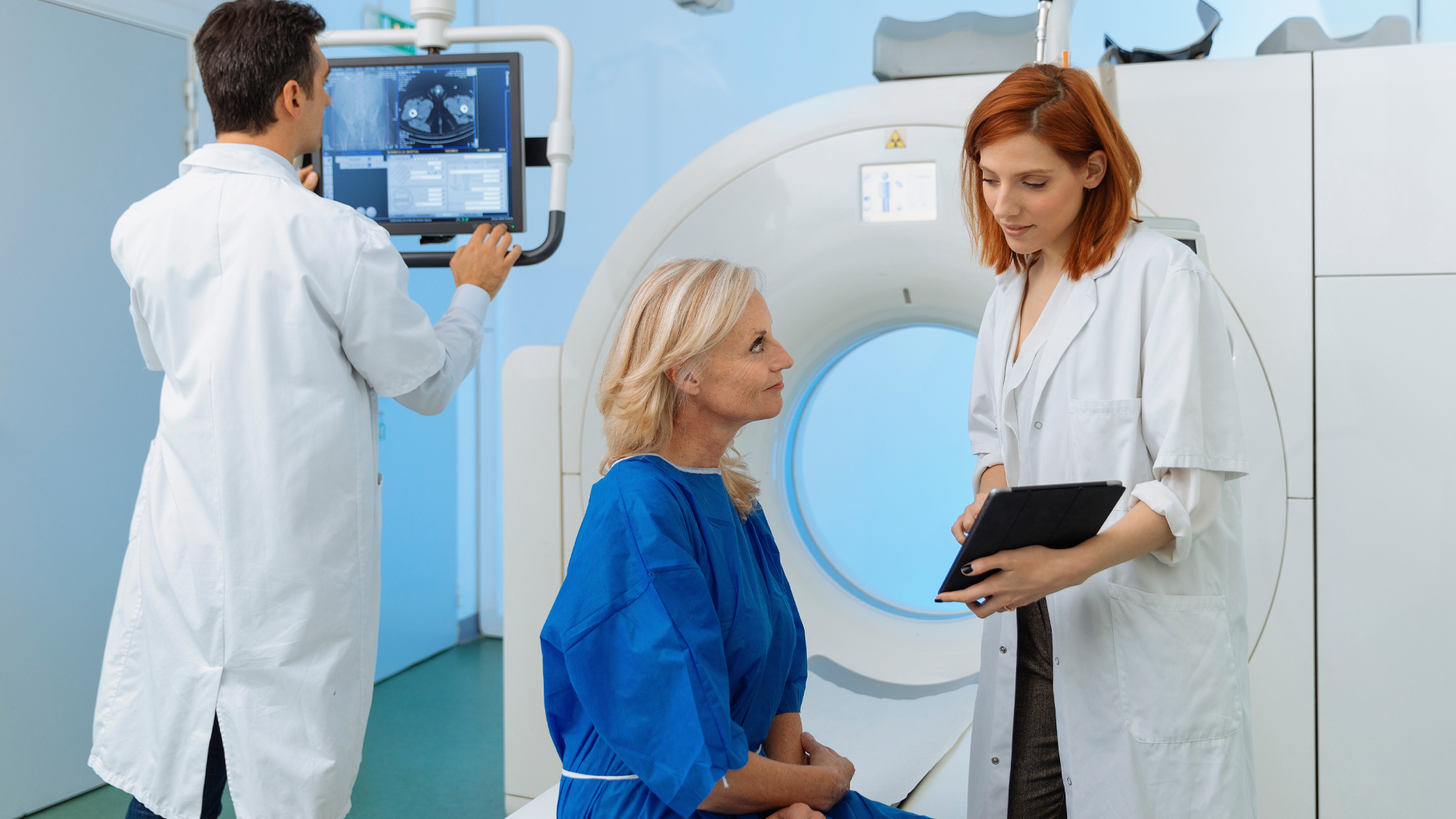
Final OVHIPEC-1 Analysis Supports HIPEC with Interval Cytoreductive Surgery in Stage III Ovarian Cancer
Hyperthermic intraperitoneal chemotherapy, also known as hot chemotherapy bath, showed a long-term overall survival improvement in patients with stage III primary epithelial ovarian cancer.
Final OVHIPEC-1 Analysis Supports HIPEC with Interval Cytoreductive Surgery in Stage III Ovarian Cancer
A 10-year follow-up of the OVHIPEC-1 trial (NCT00426257) showed a sustained progression-free survival (PFS) and overall survival (OS) benefit with hyperthermic intraperitoneal chemotherapy (HIPEC) in addition to cytoreductive surgery vs surgery alone, in patients with stage III primary epithelial ovarian cancer. The findings were published in The Lancet Oncology.1
Results from long-term follow-up showed that 93% (n = 144) of patients who received surgery alone experienced recurrence, progression or died. Comparatively, 89% (n = 109) of patients who received HIPEC in addition to surgery either progressed, had disease recurrence, or died.
The median progression-free survival between the 2 groups was 10.7 months (95% CI, 9.6-12.0) vs 14.3 months (95% CI, 12.0-18.5), yielding a hazard ratio of 0.63 (95% CI, 0.48-0.83).
The median OS was 33.3 months (95% CI, 29.0-39.1) vs 44.9 months (95% CI, 38.6-55.1), respectively (HR 0.70; 95% CI, 0.53-0.92).
“These updated survival results confirm the long-term survival benefit of HIPEC in patients with primary stage III epithelial ovarian cancer undergoing interval cytoreductive surgery,” Willemien J. van Driel, MD, PhD, of the Department of Gynecologic Oncology, Netherlands Cancer Institute, Amsterdam, and coinvestigators wrote in the study.
Study Methodology
OVHIPEC-1 is an open-label, randomized-controlled, phase 3 trial that was conducted across 8 HIPEC centers in the Netherlands and Belgium. Participants needed to be between 18 and 76 years of age, have received at least 3 cycles of neoadjuvant carboplatin plus paclitaxel, normal blood counts, adequate renal function, and a WHO performance status between 0 and 2.
Investigators randomly assigned patients 1:1 to either undergo interval cytoreductive surgery without HIPEC (surgery group) or with HIPEC (100 mg/m2 cisplatin; surgery-plus-HIPEC group). A web-based application procedure was used for randomization. Patients were allocated to a treatment group at the time of surgery, when their residual disease was anticipated to be smaller than 10 mm in diameter.
PFS was the trial’s primary end point, with OS representing the secondary end point.
Contention Around Treatment Approach
International guidelines on the use of HIPEC in primary ovarian cancer are mixed, and there remains ongoing controversy surrounding this approach.1,2
Previous reports from the OVHIPEC-1 showed a benefit with this approach. But another similarly designed trial from Korea failed to demonstrate a similar benefit. According to many clinicians, there is still limited trial data supporting this approach over other tactics.2
HIPEC is an aggressive treatment approach. First, an aggressive surgical procedure is performed, during which time all visible tumors are removed. Once the tumor has been removed, the empty space or cavity is filled with a heated chemotherapy agent. For this reason, it is also commonly known as a hot chemotherapy bath. The idea behind this is that cancer cells are less able to handle heat than healthy cell are, making it easier for the chemotherapy drugs to penetrate and kill cancer cells.2
According to the study authors, this is the first long-term survival analysis for HIPEC in ovarian cancer. However, they report that future research should focus on refining treatment selection and exploring its combination with other treatment modalities.1
References
- Aronson SL, Lopez-Yurda M, Koole SN, et al Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): final survival analysis of a randomised, controlled, phase 3 trial. Lancet. 2023;24(10):P1109-1118. doi.org/10.1016/S1470-2045(23)00396-0
- Markman M. Is HIPEC a viable treatment option for ovarian cancer? City of Hope. May 4, 2022. Accessed October 5, 2023. https://www.cancercenter.com/community/blog/2022/05/hipec-ovarian-cancer
FDA Grants Full Approval to Mirvetuximab Soravtansine for Pretreated Gynecologic Cancers
March 22nd 2024Mirvetuximab soravtansine received full approval from the FDA for pretreated adult patients with FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.