Colorectal Cancer Screening: Education, Advocacy, and Action
It is essential to continue to develop and incorporate educational strategies to increase screening for colorectal cancer in our communities.
Krista Gomez, MSN, BSN, RN, FNP-C

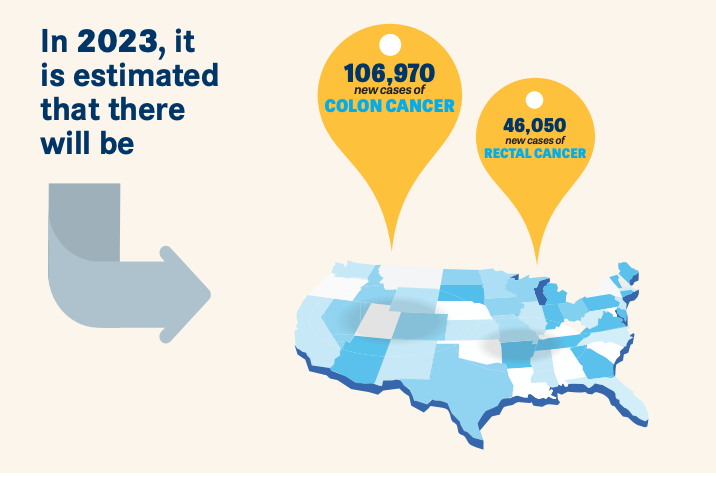
In the United States, colorectal cancer (CRC) is the third most common cancer diagnosed and second cause of cancer-related deaths.1 In 2023, it is estimated that there will be 106,970 new cases of colon cancer and 46,050 new cases of rectal cancer.1 With increased awareness and CRC screening in older adults, the overall incidence has dropped by about 1% annually from 2011 to 2019. In young adults, however, CRC has been steadily increasing by approximately 1% to 2% yearly since the mid-1990s.1 Identifying those at higher-than average risk, optimizing patient education, and recognizing alarm symptoms are key components in CRC prevention.
High-Risk Cancer Surveillance
There are many factors that increase a patient’s risk for developing CRC. These factors include high-risk polyps, genetic syndromes, and family history.
After high-quality colonoscopy, patients with polyps removed are risk stratified based on histopathology, size, location, and number of polyps removed. High-risk polyps have 1 or more of the following characteristics: an adenoma > 10 mm, villous features on histology, or pathologic findings of high-grade dysplasia.2 Patients with a highrisk colon polyp removed on initial baseline colonoscopy have a 2- to 4-fold increased risk of developing CRC.2 Additionally, patients identified to have a sessile serrated polyp > 10 mm should be managed as having a high-risk polyp, as this type of polyp is associated with a 3-fold increased incidence of CRC within 3 to 5 years.2 Alternatively, patients with low-risk polyps (1-2 tubular adenomas, < 10 mm) or no polyps removed on initial colonoscopy are considered low risk for developing CRC, with a recommended follow-up colonoscopy in 7 to 10 years.3
Genetic Syndromes
Approximately 5% to 10% of CRC diagnoses are secondary to inherited cancer syndrome. Lynch syndrome is the most common cause of hereditary CRC, accounting for 2% to 3% of new cases diagnosed.4 It is an autosomal dominant inheritance that is secondary to mutations in the DNA mismatch

repair gene (which repairs mistakes in DNA replication). Normally these genes protect against certain cancers; however, this mutation alters the normal function of 5 genes (MLHL, MSHE, MSH6, PMS2, and EPCAM), increasing risk for CRC as well as extracolonic malignancy. Patients with Lynch syndrome can have an up to 80% lifetime risk of CRC.5 Many organizations, including the National Comprehensive Cancer Network (NCCN), American College of Gastroenterology, and US MultiSociety Task Force on Colorectal Cancer, have developed guidelines on the management and screening of CRC in these high-risk individuals. Guidelines for high-risk screening advise starting colonoscopy at the ages of 20 to 25 (or 5 years earlier than the youngest family member if diagnosed younger than age 25) and repeating at intervals of every 1 to 2 years.4 Management of extracolonic malignancies can be found at the NCCN website. Familial adenomatous polyposis (FAP) is the second most common autosomal dominant inherited cancer syndrome and accounts for 1% of CRC.6 This is a result of a mutation in the APC gene, a tumor-suppressor gene.6 Patients with FAP can develop thousands of colorectal polyps with a nearly 100% overall lifetime risk for CRC development.6 Attenuated FAP is a milder form of classic FAP and typically presents at an older age with development of fewer polyps but still with 70% lifetime risk of CRC development.6 Management of patients with FAP includes annual colonoscopy starting at ages 10 to 12 and should persist unless polyp burden cannot be controlled with endoscopic removal.6 Surgical management in patients with FAP involves total vs subtotal colectomy.6 If the rectum remains, continued surveillance with flexible sigmoidoscopy every 6 months is necessary to reduce rectal cancer risk.6
Additional genetic syndromes associated with higher-than-average CRC risk include Li-Fraumeni syndrome, MUTYH-associated polyposis, PeutzJeghers syndrome, serrated polyposis syndrome, and Cowden syndrome.7 Although all-cause risk of CRC in these genetic syndromes is less common than in Lynch syndrome and FAP, it is important to be aware of cancer risk reduction strategies for these patients, which can be located on the NCCN website
Family History
Acquiring a detailed family history can help health care professionals identify patients who are at a higher-than-average risk of CRC. More intensive screening strategies should be considered for individuals who have a family history of CRC in multiple relatives, who have relatives diagnosed younger than age 50, who have a first-degree relative (FDR) with an advanced adenoma, who have inflammatory bowel disease, or who have 1 of the aforementioned inherited cancer syndromes.8
A cross-sectional study was completed to evaluate advanced adenomas and/or adenocarcinoma in patients with an FDR with CRC (high risk) compared with those with no family history (average risk). The results showed that in the population of patients with an FDR with CRC, 11.3% had advanced neoplasia. In comparison, advanced neoplasia was identified in 6.3% of the control patients.8 Historically, the overall lifetime risk of developing CRC in individuals with familial CRC increases 2- to 6-fold. These patients should start screening with high-quality colonoscopy at the age of 40 or 10 years prior to FDR diagnosis of an advanced adenoma or malignancy, whichever comes first. Follow-up colonoscopy should be completed at a maximum of 5-year intervals.9
Alarm Symptoms
Symptoms that can be indicative of CRC include rectal bleeding, new-onset anemia, changes in bowel habits, or incomplete evacuation.10 For those patients who have been diagnosed with CRC, approximately 15% to 74% experience rectal bleeding.10 Unfortunately, rectal bleeding is often underreported, leading to a delay in diagnosis. Attributing bleeding to a benign cause, embarrassment, or concern about diagnostic procedures are all factors that lead to underreporting symptoms.11 Assessment and recognition of alarm symptoms may promote a timely evaluation.
Optimizing Education
Although CRC awareness and prevention strategies continue to improve, CRC remains the third most common cancer diagnosis.1 Optimizing education strategies is essential to reduce morbidity and mortality from this disease. Patient education should include discussions on risk-reduction strategies, prevention, and screening modalities. It should also seek to address barriers to screening and affordability. Utilization of screening questionnaires, providing education brochures/pamphlets, presentations, and providing links or QR codes to online resources can all be beneficial to enhance knowledge and awareness. I recommend leveraging the American College of Gastroenterology, as it is one of the many associations that provide educational resources on their websites for utilization and patient education.
Additionally, continuing education and reinforcement for the medical community are necessary to identify and capture those individuals with higher-than-average risk based on family history or prior advanced neoplasia. The CDC has developed continuing education courses for providers to increase and improve CRC screening.12 To improve accessibility, gastroenterology practices in local communities are providing direct access to screening colonoscopies without the need for a consult. Compiling a list of practices that incorporate direct access can reduce the inconvenience or time limitations that often prevent screening.
It is also important to discuss that for average-risk patients, there are stool-based screening tests such as fecal immunochemical testing and stool DNA testing. Computed tomography colonography can be offered as an intraluminal imaging modality, often referred to as a virtual colonoscopy. Pros and cons of each test should be discussed. It should be emphasized that any screening modality is better than not screening at all.13
It is essential to continue to develop and incorporate educational strategies to increase screening for CRC in our communities.
References
- Key statistics for colorectal cancer: how common is colorectal cancer? American Cancer Society. Revised January 13, 2023. Accessed January 30, 2023. bit.ly/3NlpiWS
- He X, Hang D, Wu K, et al. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology. 2020;158(4):852-861.e4. doi:10.1053/j.gastro.2019.06.039
- Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91(3):463-485.e5. doi:10.1016/j.gie.2020.01.014
- Rubenstein JH, Enns R, et al. American Gastroenterological Association institute guideline on the diagnosis and management of Lynch syndrome. Gastroenterology. 2015;149(3): 777-782. doi:10.1053/j.gastro.2015.07.036
- Bhattacharya P, McHugh TW. Lynch Syndrome. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK431096/
- Carr S, Kasi A. Familial Adenomatous Polyposis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK538233/
- Rebuzzi F, Ulivi P, Tedaldi G. Genetic predisposition to colorectal cancer: how many and which genes to test? Int J Mol Sci. 2023;24(3):2137. doi:10.3390/ijms24032137
- Armelao F, Paternolli C, Franceschini G, et al. Colonoscopic findings in first-degree relatives of patients with colorectal cancer: a population-based screening program. Gastrointest Endosc. 2011;73(3):527-534.e2. doi:10.1016/j.gie.2010.12.025
- Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331(25):1669-1674. doi:10.1056/NEJM199412223312501
- Tong GX, Chai J, Cheng J, et al. Diagnostic value of rectal bleeding in predicting colorectal cancer: a systematic review. Asian Pac J Cancer Prev. 2014;15(2):1015-1021. doi:10.7314/apjcp.2014.15.2.1015
- Pedersen AF, Hansen RP, Vedsted P. Patient delay in colorectal cancer patients: associations with rectal bleeding and thoughts about cancer. PLoS One. 2013;8(7):e69700. doi:10.1371/journal.pone.0069700
- Colorectal cancer screening continuing education. Centers for Disease Control and Prevention. March 14, 2022. Accessed February 5, 2023. https://bit.ly/3qQMXXY
- Jin J. Screening tests for colorectal cancer. JAMA. 2016;315(23):2636. doi:10.1001/jama.2016.7616

Early Data May Show PFS Benefit With Cancer Vaccine in Metastatic CRC
April 13th 2024Patients with metastatic microsatellite stable colorectal cancer treated with a personalized neoantigen cancer vaccine after chemotherapy demonstrated an early trend towards a progression-free survival benefit.