Tackling the Adverse Events of Immunotherapy
Most patients who receive immunotherapies will experience few, if any, treatment-related adverse effects (AEs). But some patients receiving these treatments can experience serious AEs caused by an overactivation of the immune system and its effects on healthy cells.
Igor Puzanov, MD, MSCI, FACP

Immunotherapy agents represent a transformational advance in our ability to treat many cancers. Rather than introducing a therapy that kills both healthy and malignant cells, immunotherapy agents harness the body’s natural ability to recognize and control the invasive cells that can form tumors.
Most patients who receive immunotherapies will experience few, if any, treatment-related adverse effects (AEs). But some patients receiving these treatments can experience serious AEs caused by an overactivation of the immune system and its effects on healthy cells.
It is important for clinicians to identify AEs quickly and assign such toxicities an appropriate grade using the standard Common Terminology Criteria for Adverse Events 5.0 grading system. Based on that grading, treatment should be designed using standard guidelines developed by oncology societies, including the Society for Immunotherapy of Cancer and American Society of Clinical Oncology (ASCO). Most of the toxicities caused by immunotherapy drugs are reversible by using steroid or other immunosuppressive therapies.
It is critical to ensure clinicians recognize and know how to manage these treatment-emergent AEs so patients can seek appropriate medical attention quickly and continue to take advantage of the benefits of immunotherapy.
Common AEs of Immunotherapy
Several immunotherapy drugs have been approved by the FDA to treat various types of advanced cancers. Approved immunotherapies include CTLA-4 inhibitor ipilimumab (Yervoy); PD-1 inhibitors pembrolizumab (Keytruda), nivolumab (Opdivo), and cemiplimab (Libtayo); and PD-L1 inhibitors atezolizumab (Tecentriq) and avelumab (Bavencio) (TABLE 1).1-6
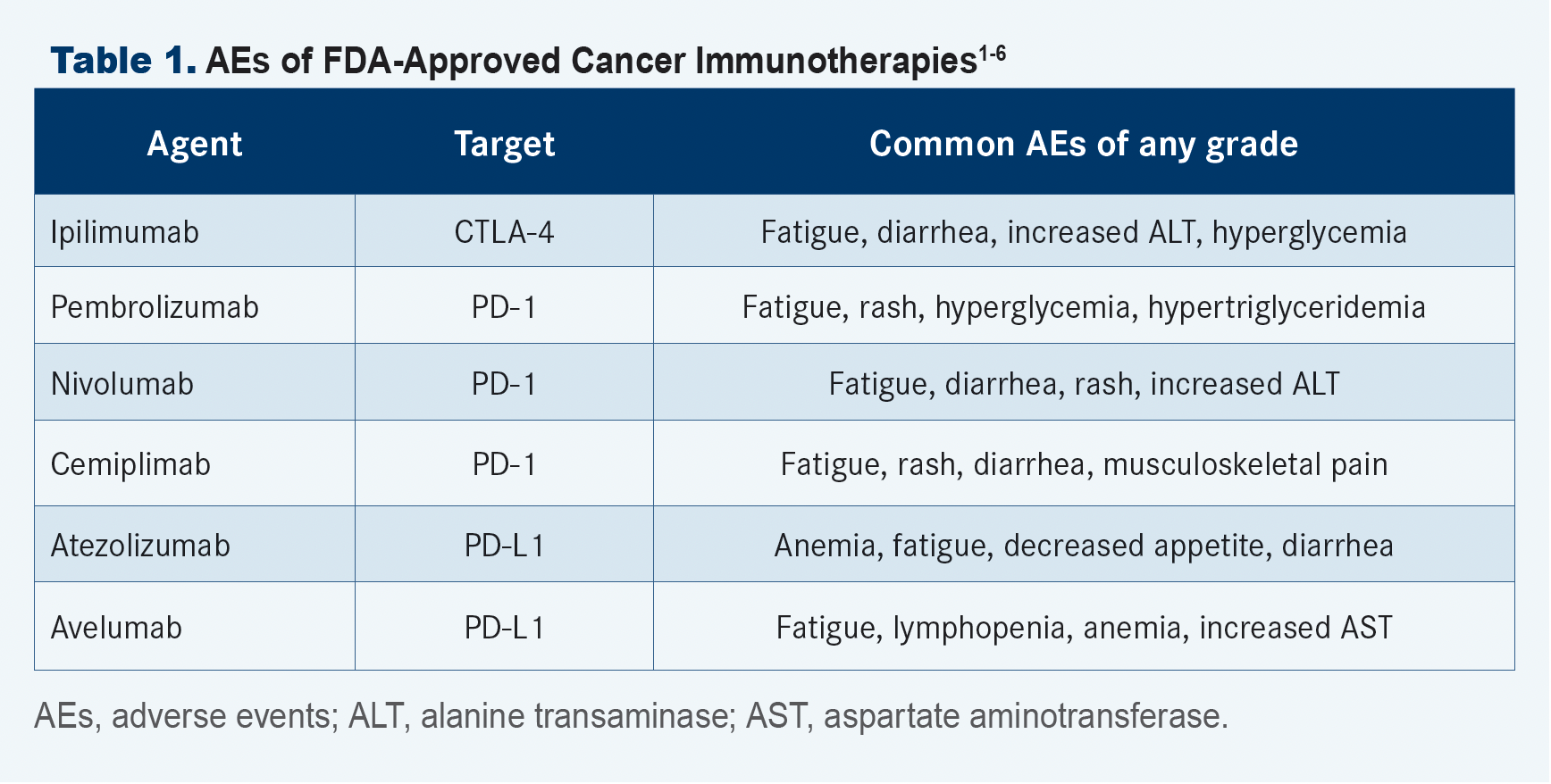
Immunotherapy AEs can be difficult to identify because they can cause symptoms that are associated with common ailments and infections. Some of these adverse reactions might resemble another medical issue but require a very different course of action. For example, pneumonitis, which a small number of patients on checkpoint inhibitor immunotherapy may experience, can cause some of the same symptoms as viral or bacterial respiratory infection but should be treated with steroids instead of antibiotics. The primary immunotherapy-related AEs patients and clinicians should be aware of include flu-like symptoms, asthenia, arthralgias, and rash. Endocrinopathies such as thyroiditis, hypophysitis, adrenal insufficiency, and type I diabetes must be recognized quickly, as these complications may lead to the need for lifelong hormonal replacement therapy.
There have been reports of inflammation of organs, including pneumonitis, pancreatitis, hepatitis, nephritis, colitis, dermatitis (redness, itchiness, or rash), neuritis, uveitis, and myocarditis. Although myocarditis occurs in fewer than 1% of patients receiving immunotherapy, it is likely the most serious adverse reaction that can occur because of these agents.7 Early detection of this potentially fatal AE, which carries a 40% to 50% risk of death, is critical, but there are currently no clear ways to accurately predict or identify this type of immunotherapy-associated toxicity.7
I was recently part of a multidisciplinary team at Roswell Park Comprehensive Cancer Center (Roswell Park) that identified troponin, a protein that enters the bloodstream only when the heart is damaged, as an early and reliable predictor of myocarditis in patients with cancer receiving immunotherapy with immune checkpoint inhibitors.7
Findings of this retrospective study, which reviewed the records of 1001 patients with cancer who were treated with 1 or more immunotherapy agents at Roswell Park from 2016 to 2020, suggested that weekly troponin monitoring during the first 6 weeks of treatment could help physicians detect this rare but potentially fatal AE of immunotherapy. Any patient showing an elevation in troponin levels or signs of cardiotoxicity, such as angina or arrhythmia, should be referred to a cardio-oncology team for complete evaluation, including electrocardiogram, echocardiogram, or cardiac MRI.
Although immunotherapy-related myocarditis is extremely rare, it occurs with greater frequency and severity when immunotherapy drugs are given in combination, and early recognition is critical. For example, in a multicenter analysis, my colleagues and I identified 2 cases of myocarditis that developed in patients with metastatic melanoma following treatment with a combination of ipilimumab and nivolumab, which are 2 commonly used immune checkpoint inhibitors.8
Treatment of Immunotherapy AEs
Reduction of immunotherapy-related AEs relies heavily on corticosteroids and other immunomodulatory agents, which should be prescribed to reduce the potential for short-term and long-term complications. It remains unclear whether prophylactic antibiotics should routinely be prescribed to reduce the potential for opportunistic infection in patients receiving steroids. Treatment should be individualized depending on each patient’s medical history, comorbidities, and underlying disease status, as well as the type, number, and severity of AEs.
Guidance for Medical Professionals
Because immunotherapy is a relatively new treatment option, many of these agents have AEs that have not been previously observed. AEs stemming from immunotherapy use can affect the skin, lungs, gastrointestinal and endocrine systems, joints, heart, and other organs. Some of these AEs are just beginning to be described, therefore clinicians need guidance on how to recognize early signs, how to treat AEs, and when to refer a patient to a disease specialist.
For this reason, I have been helping to create guidance for medical professionals who care for patients receiving immunotherapy drugs. Along with other experts in this field, I helped write the first consensus guidelines on how to recognize and respond to immunotherapy-related AEs and am contributing to similar resources to be issued by ASCO and the National Comprehensive Cancer Network (TABLE 2).9
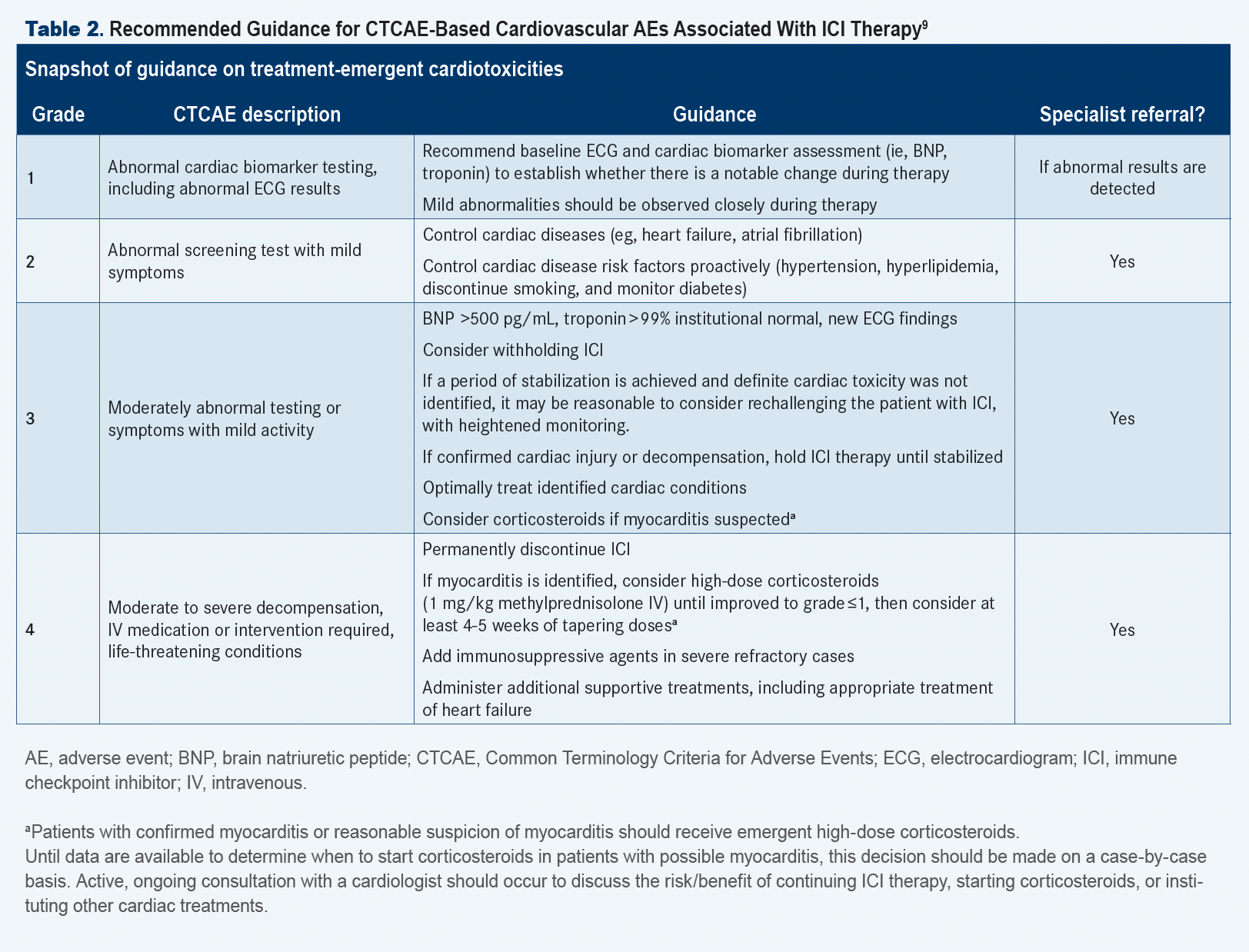
Information is key in identifying and managing any AEs of immunotherapy. It is critical for medical professionals to give patients clear written guidance before a course of immunotherapy is started and to keep that flow of information going back and forth during clinic visits and between appointments.
When my patients on immunotherapy ask me about possible AEs, I stress that immunotherapy is an incredibly important and effective option for treating many cancers and that AEs are rare. I also strongly encourage my patients to become active participants in their treatment by learning about these possible AEs, monitor for these adverse reactions, and respond to any symptoms that develop. Together, we can help to ensure these new and advanced treatments are as effective as possible.
References
- Yervoy. Prescribing information. Bristol Myers Squibb; 2022. Accessed April 7, 2022. bit.ly/38px6Go
- Keytruda. Prescribing information. Merck Sharp & Dohme Corp; 2022. Accessed April 7, 2022. bit.ly/3ul9M52
- Opdivo. Prescribing information. Bristol Myers Squibb; 2022. Accessed April 7, 2022. bit.ly/3Klihmx
- Libtayo. Prescribing information. Regeneron Pharmaceuticals Inc; 2021. Accessed April 7, 2022. bit.ly/3DSfK0D
- Tecentriq. Prescribing information. Genentech; 2022. Accessed April 7, 2022. bit.ly/3Kof1GP
- Bavencio. Prescribing information. EMD Sereno, Inc; 2019. Accessed April 7, 2022. bit.ly/3raRCBe
- Puzanov I, Subramanian P, Yatsynovich YV, et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J Immunother Cancer. 2021;9(6):e002553. doi:10.1136/jitc-2021-002553
- Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722-730. doi:10.1056/NEJMoa1805453
- Puzanov I, Diab A, Abdallah K, et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z




