Caring for a Patient With Relapsed Mantle Cell Lymphoma Undergoing CAR T-Cell Therapy
CAR T-cell therapy represents a viable treatment option for many patients, but there are potentially serious adverse events.
Nancy C. Long, MSN, AGACNP-BC

Historically, patients with relapsed and/or refractory (R/R) large B-cell lymphoma had few viable treatment options. However, when chimeric antigen receptor (CAR) T-cell therapy received FDA approval in 2017, it offered a novel approach for many of these patients. By 2018, tisagenlecleucel (Kymriah) was approved for patients with R/R large B-cell lymphoma, further expanding CAR T-cell therapy’s reach.1
CAR T-cell therapy involves collecting a patient’s T cells through leukapheresis. CAR-coding viral DNA is incorporated into these cells at the manufacturing facility, transforming them into CAR T cells. Prior to receiving the cells, patients have a small amount of chemotherapy to allow for CAR T-cell expansion. These cells are then infused back into the patient where they bind and subsequently kill the cancer cells.2 Although CAR T-cell therapy has created an avenue for remission and a potential cure for some patients, it is important to carefully evaluate and educate patients because the therapy can have severe adverse effects.
Patient Case
A 50-year-old man, “Mr D,” presented to the emergency department in summer 2014 with fatigue, 20-lb weight loss, and notable inguinal and submandibular adenopathy. An inguinal biopsy showed that Mr D had mantle cell lymphoma (MCL). At the time of diagnosis, his bone marrow biopsy also showed evidence of lymphoma. He received a diagnosis of stage IV MCL. Initial treatment included hyperfractionated CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone, plus methotrexate and cytarabine; hyper-CVAD). In early 2015, he was found to be in complete remission and underwent autologous peripheral blood stem cell transplant.
In fall 2016, Mr D relapsed; he again had notable adenopathy, below and above the diaphragm. He began treatment with the Bruton tyrosine kinase inhibitor ibrutinib (Imbruvica). He tolerated treatment with ibrutinib well until summer 2021 when he again experienced disease progression. He then transitioned to treatment with obinutuzumab (Gazyva) and venetoclax (Venclexta), but this approach was followed by rapid disease progression in fall 2021.
After 4 treatment modalities failed, it was clear that Mr D would need a different approach, and CAR T-cell therapy was considered. To minimize potential adverse effects, the team administered rituximab (Rituxan) and hyper-CVAD to Mr D to debulk his tumor burden. However, when Mr D underwent a PET scan prior to receiving CAR T-cell therapy, it showed that his adenopathy had increased and his Deauville score was 5. Mr D received lymphodepleting chemotherapy and CAR T-cell infusion in January 2022.
He tolerated the infusion well, but on day 6 post infusion, he had a fever of 102 °F and was admitted to the hospital. His laboratory results included a white blood count of 0.2 × 103 /mcL, absolute neutrophil count 300 × 103/mcL, ferritin 722 ng/mL, uric acid 2.9 mg/dL, C-reactive protein (CRP) 18.9 mg/L, and lactate dehydrogenase (LDH) 167 unit/L. He continued to be febrile for more than 24 hours and became hypotensive. Mr D had grade 2 cytokine release syndrome (CRS).
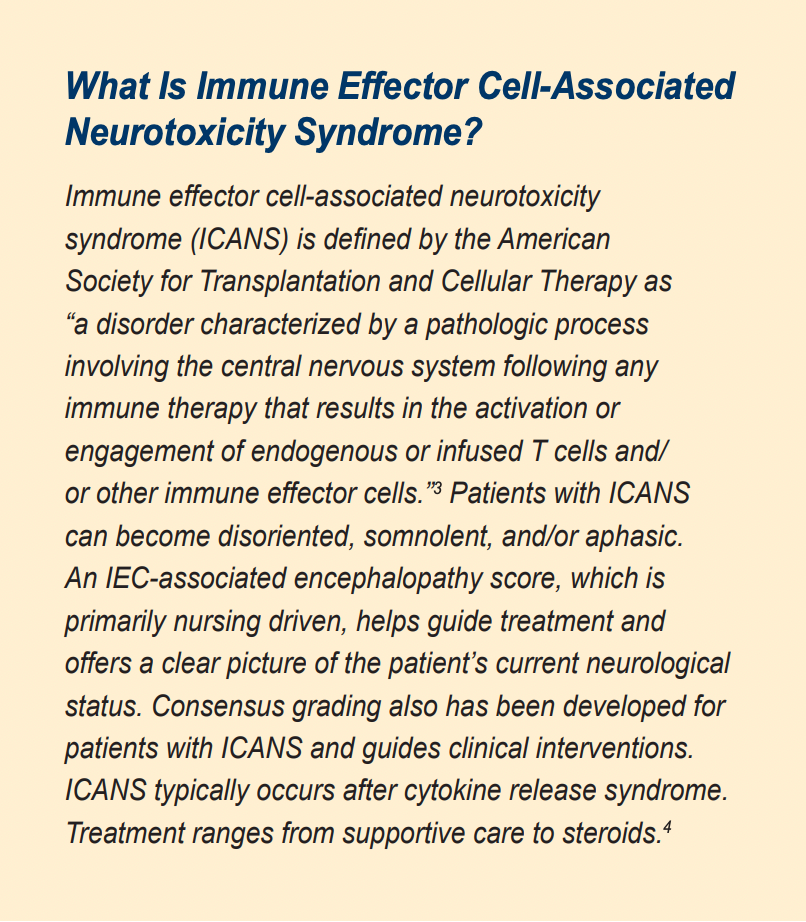
He received supportive care as well as treatment with tocilizumab (Actemra). He subsequently defervesced. Yet on day 11 post infusion, he was found wandering the hospital unit, disoriented to month and year. He was unable to write a sentence well or count backward from 100. His laboratory results were notable; ferritin 1051 ng/mL, CRP 150.9 mg/L, and LDH 198 unit/L. He was diagnosed with grade 2 immune effector cell-associated neurotoxicity syndrome (ICANS). He received dexamethasone and quickly improved. Steroids were tapered over 48 hours with resolution of his symptoms. He was discharged on day 14 and followed closely in the outpatient clinic.

Mr D had a PET scan on day 30. He had responded to treatment with decreased adenopathy and had a Deauville score of 3. By his 3-month scan post CAR T-cell infusion, he was in complete remission.
Implications for Practice
CRS and ICANS can be potentially severe adverse events (AEs) of CAR T-cell therapy. Patients must be carefully screened and evaluated for this treatment. Organ function testing must be adequate, and patients must have a strong support system because of the potential severity of AEs. However, for patients with high-risk disease and for those who have not responded to multiple lines of therapy, CAR T-cell therapy represents a viable treatment option.
References
- Kymriah (tisagenlecleucel), first-in-class CAR-T therapy from Novartis, receives second FDA approval to treat appropriate r/r patients with large B-cell lymphoma. Novartis. News release. May 1, 2018. Accessed October 21, 2022. https://bit.ly/3z1QOlZ
- CAR T-cell therapy. NCI. Accessed October 21, 2022. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758
- Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34-48. doi:10.1016/j.annonc.2020.10.478




