
Holly Chitwood, DNP, APRN, FNP-C, AGACNP-BC, shares her real-world experience with circulating-tumor-DNA monitoring in the colorectal cancer setting.

Lindsay Fischer is an editor at ONN. She joined MJH life sciences in 2021 after graduating from Rutgers University with majors in both English and Spanish and a minor in creative writing. When she is not writing, or researching the latest oncology nursing news, Lindsay is an amateur backpacker and rollerblader. Email Lindsay at lfischer@mjhlifesciences.com

Holly Chitwood, DNP, APRN, FNP-C, AGACNP-BC, shares her real-world experience with circulating-tumor-DNA monitoring in the colorectal cancer setting.

Data from a single-institution study showed that 98.6% of their patients with head and neck cancer experienced oral mucositis.

Those receiving dostarlimab experienced numerical improvements from baseline in terms of quality of life, emotional functioning, pain, and back and pelvis pain scores.
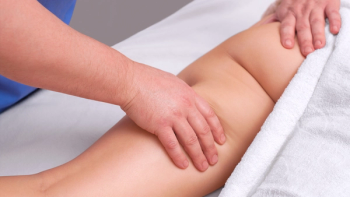
Melanie Taylor, APRN, and Mark Lin, APRN, share how they optimize strategies in caring for patients with lymphedema.

Proper counseling is needed to help decrease fertility-related psychological distress in adolescent/young adult cancer survivors.

Advanced practice providers were more likely to be impacted by prior authorizations than their physician colleagues, according to survey responses.

Beth Sandy, MSN, CRNP, and Tajuana Bradley, MSN, APRN-BC, discuss the importance of molecular testing in non–small cell lung cancer.

The FDA has expanded the indication for enzalutamide to include patients with high-risk nonmetastatic castration-sensitive prostate cancer.
Tammy Triglianos, DNP, ANP-BC, AOCNP, discusses optimal adjuvant treatment approaches for patients with stage III colon cancer.

The FDA has approved capivasertib plus fulvestrant to treat patients with locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN-alterations.

The FDA has approved pembrolizumab plus chemotherapy to treat adults with locally advanced or metastatic, HER2-negative gastric cancers.

Following the pandemic, rates of violence in health care are still high.

Nicole Gay, APRN-C, shares how nurse navigators can talk to their patients about lung cancer screening.

Like patients, caregivers need education and support, but are often forgotten or unheard.

A phase 3 trial based out of India suggests that olanzapine may be useful in reducing chemotherapy-induced nausea and vomiting.

Patients who were in the intervention arm were 67% less likely to go to the emergency department in the last days of their life.

The Undiagnosed Cancer Clinic, or UCC, was established with the goal of reducing the time that patients wait for their initial oncology consultation appointments.

Screening for financial toxicity should be routine in cancer care, according to investigators.

Incorporating frailty screenings into preexisting workflows may be an effective way to provide more holistic care to patients with head and neck cancer.

Investigators reflect on the Oncology Care Model and its implications.

Freshta Poupal, RN, and Frannie Bell, NP, share their experiences with teclistamab in clinical practice.

An initiative led by nurses helped reduce operating-room pressure injuries and saved their institution an estimated $80,210 per year.

The FDA has approved pembrolizumab in combination with chemotherapy as a treatment for patients with locally advanced unresectable or metastatic biliary tract cancer.

Holly Chitwood, DNP, FNP-C, AGACNP-BC, explains how circulating tumor DNA monitoring helps providers screen minimal residual disease in individuals with colorectal cancer.

The Oncology Care Model did not significantly improve care, yet patients were satisfied with their care and many oncologists overcame their hesitancy towards value-based models.

In a survey, 75% of Black respondents said that racism had impacted their mental health in the workplace.

The FDA has approved toripalimab, with cisplatin and gemcitabine, and as a monotherapy, to treat adults with nasopharyngeal carcinoma.

Ivosidenib, a targeted therapy, has been approved for patients with relapsed or refractory myelodysplastic syndromes and an IDH1 mutation.

Adjuvant alectinib significantly improved disease-free survival in patients with ALK-positive, early-stage, non–small-cell lung cancer.

The Health Resources and Services Administration has announced that it will be investing in the training of new nurses and growing the workforce.