
The FDA expanded the approval of methotrexate to include patients with pediatric acute lymphoblastic leukemia and polyarticular juvenile idiopathic arthritis.

The FDA expanded the approval of methotrexate to include patients with pediatric acute lymphoblastic leukemia and polyarticular juvenile idiopathic arthritis.

CE lesson worth 1 contact hour that is intended to advanced practice nurses, registered nurses, and other healthcare professionals who care for patients with cancer.
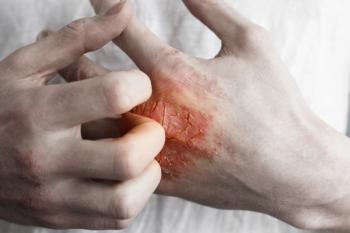
Although Stivarga may cause side effects after initial treatment, a dose escalation strategy and the preemptive use of steroid creams may help patients to tolerate the regimen better, while allowing them to capitalize on the agent’s survival benefit.

CE lesson worth 1 contact hour that is intended to advanced practice nurses, registered nurses, and other healthcare professionals who care for patients with cancer.

CE lesson worth 1 contact hour that is intended to advanced practice nurses, registered nurses, and other healthcare professionals who care for patients with cancer.

CE lesson worth 1 contact hour that is intended to advanced practice nurses, registered nurses, and other healthcare professionals who care for patients with cancer.
Evaluating the benefits of the subQ vs IV formulation of the trastuzumab-pertuzumab combination for the management of HER2+ breast cancer and examining the potential use of at-home administration.


CE lesson worth 1 contact hour that is intended to advanced practice nurses, registered nurses, and other healthcare professionals who care for patients with cancer.

The FDA has approved pembrolizumab (Keytruda) or patients with recurrent or metastatic cutaneous squamous cell carcinoma that is not curable by surgery or radiation.

CE lesson worth 1 contact hour that is intended to advanced practice nurses, registered nurses, and other healthcare professionals who care for patients with cancer.

The FDA has granted a Fast Track designation to CLR 131 for use as a treatment for patients with lymphoplasmacytic lymphoma (LPL)/Waldenstrom macroglobulinemia (WM) who have received at least 2 prior treatment regimens.

This OncLive® webinar focused on the impact of COVID-19 on oncology nursing care for patients with hepatocellular carcinoma. We featured oncology nurse experts, Amy Hillsman, CRNP, and Abigail Smith, CRNP, discussing important topics and considerations for nurses and how they continue to provide the best care for their patients during this time.


The FDA has accepted a biologics license application (BLA) for sacituzumab govitecan as a treatment for patients with metastatic triple-negative breast cancer (TNBC) who have received at least 2 prior therapies for metastatic disease, according to a statement from the company developing the antibody-drug conjugate (ADC), Immunomedics.


In 2018, the V Foundation awarded $25 million for cutting-edge cancer research.

New initiatives boost awareness and fundraising: Kicks to Beat Cancer, Craig Sager Style Day, Blizzard/Overwatch League Season Preview and WeRateDogs “H*ck Cancer” campaign.

Funds matched by Bristol-Myers Squibb for a total of $1.3 Million to be used for Breakthrough Cancer Research.

Oncology Nursing News®, a digital and print media enterprise dedicated to bringing the oncology nursing community together by providing them with the latest nursing news, clinical insights and resources, welcomes registered nurse Deborah A. Boyle as its editor in chief following the retirement of Lisa Schulmeister.

Scientists, physicians and patients collaborate to improve treatment for rare cancer.

The Aplastic Anemia & MDS International Foundation (AAMDSIF), the world's leading non-profit health organization dedicated to serving patients afflicted with bone marrow failure disease, announced today that Neil Horikoshi has been named the Foundation's new Chief Executive Officer and Executive Director.
The combination use of adjuvant dabrafenib (Tafinlar) plus trametinib (Mekinist) has continued to show a significant relapse-free survival benefit among patients with resected stage III BRAF-mutant melanoma.

Patients with advanced cancers associated with NTRK gene fusions showed an 80% objective response rate (ORR) with larotrectinib, a TRK inhibitor, according to pooled results from 3 small trials.
Frontline alectinib (Alecensa), a next-generation tyrosine kinase inhibitor, enabled Asian patients with untreated ALK-positive advanced non–small cell lung cancer (NSCLC) to live significantly longer without disease progression compared with those who were treated with crizotinib (Xalkori) as initial therapy.

The FDA has granted lorlatinib (Lorbrena) an accelerated approval for the treatment of patients with ALK-positive metastatic non–small cell lung cancer (NSCLC) who have progressed on 1 or more ALK tyrosine kinases inhibitors (TKIs).

The Food and Drug Administration (FDA) has granted a priority review to the oral chemotherapy TAS-102 (trifluridine/tipiracil; Lonsurf) for use in previously treated patients with advanced or metastatic gastric adenocarcinoma, including cancer of the gastroesophageal junction.

Pet dogs help find cures for cancer.

In an effort to help individuals gain access to breast health services, Barbells for Boobs and CancerCare®, the leading national cancer support organization, have officially launched the NYC Support Line.
Published: May 28th 2021 | Updated:

Published: March 31st 2018 | Updated:
Published: July 6th 2018 | Updated:

Published: August 9th 2018 | Updated:

Published: July 2nd 2018 | Updated:

Published: July 13th 2018 | Updated: