
Criteria for progression-free and overall survival endpoints in patients with endometrial cancer with mismatch repair proficient disease were not met.

Criteria for progression-free and overall survival endpoints in patients with endometrial cancer with mismatch repair proficient disease were not met.

Trial data support potential treatment options with durvalumab-based regimens for advanced or recurrent endometrial cancer.
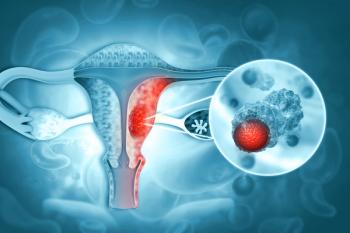
Patients across endometrial cancer subgroups derived a favorable survival benefit with pembrolizumab plus chemotherapy in the KEYNOTE-868 trial.

Patients with microsatellite instability high primary advanced or recurrent endometrial cancer derived a significant survival benefit when treated with dostarlimab plus chemotherapy.
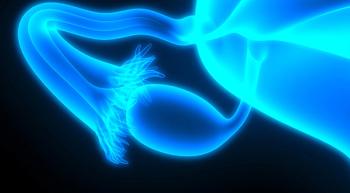
Patients with platinum-resistant or -refractory epithelial ovarian cancer treated with olaparib plus cediranib did not experience improvements in PFS and OS compared with chemotherapy.

Further evidence strengthens the support of mirvetuximab soravtansine as a new standard of care for folate receptor-alpha—positive ovarian cancer resistant to platinum chemotherapy.

Meghan K. Berkenstock, MD, discusses the growing need for strong collaboration between ophthalmologists and gynecologic oncology care teams.
Pembrolizumab inspired a 70% risk reduction in women with mismatch repair–deficient advanced endometrial cancer and a 46% risk reduction in patients with mismatch repair–proficient disease.

With a follow-up of 11.7 months, the estimated 12-month progression-free survival rate was 81% in patients who received neoadjuvant olaparib for BRCA-positive ovarian cancer.

Mary Katherine Montes de Oca, MD, discusses the potential benefit of cryocompression therapy in preventing peripheral neuropathy in patients with gynecologic cancers who are receiving chemotherapy.

Patients with ovarian cancer who received hyperthermic intraperitoneal chemotherapy with cytoreductive surgery did not experience worse health-related quality of life, according to investigators.
Dostarlimab met its primary end point in the phase 3 RUBY trial by increasing the 2-year progression-free survival rate to 36.1%, compared with 18.1% with placebo.

In the all-comer population, the overall response rate was 33.8%, including a complete response rate of 7.5% and a partial response rate of 26.3%.

Research presented at the 2023 Society of Gynecologic Oncology Annual Meeting On Women’s Health suggests that the Carolina frailty index score may be useful for women with ovarian cancer.

The gynecological cancer lymphedema questionnaire and the lower extremity lymphedema screening questionnaire showed a demonstrable correlation in total scores, according to an analysis of women with advanced endometrial cancer.

The PARP inhibitor, niraparib, in conjunction with the VEGF inhibitor, bevacizumab, resulted in encouraging responses from patients with advanced ovarian cancer.

Data collected with patient-reported outcomes show that pembrolizumab, with or without bevacizumab, is associated with a favorable toxicity profile in effectively treating women with persistent, recurrent, or metastatic cervical cancer.

Whether administered as an immune primer or concurrently with extended field chemoradiation, atezolizumab demonstrated efficacy in triggering T-cell clonal expansions and prolonging progression-free survival in patients with cervical cancer.

Selinexor demonstrated impressive efficacy in extending progression-free survival among patient with advanced or recurrent endometrial cancer, particularly among patients with wild-type p53 endometrial cancer.

The ongoing FLORA-5 trial will assess whether the addition of oregovomab to a standard chemotherapy regimen will improve progression-free and overall survival in patients with advanced epithelial ovarian cancer.

Maintenance olaparib showcased progression-free survival benefit in almost half of the patients with ovarian cancer vs 21% of those who received placebo, including consistent benefit in high- and low-risk patients.