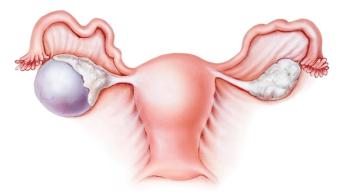
Screening for ovarian cancer in asymptomatic women who are not known to have a high-risk hereditary cancer syndrome has no benefit and may lead to harm due to false positives, according to a new review conducted by the US Preventive Services Task Force (USPSTF).