
Smoking prevention programs need to include warnings that the risk of “trying” a cigarette can lead to daily smoking, and that 69% of people in global survey data who have tried a cigarette became smokers.

Smoking prevention programs need to include warnings that the risk of “trying” a cigarette can lead to daily smoking, and that 69% of people in global survey data who have tried a cigarette became smokers.

Many adolescent and young adult (AYA) survivors of cancer end up "lost to follow-up," according to recent research. Nurses charged with patient education should take heed, and be sure to stress the importance of follow-up care to their AYA patients and their families.

The FDA has approved durvalumab (Imfinzi) for the treatment of patients with locally advanced, unresectable stage III non–small cell lung cancer (NSCLC) who have not progressed following chemoradiotherapy.
Advancements in CAR-T cell therapy are changing the way many cancers are being treated.

Agios Pharmaceuticals announced that the FDA has granted a priority review designation to its targeted therapy, ivosidenib (AG-120), for the treatment of patients with relapsed/refractory IDH1-mutant acute myeloid leukemia (AML).
Both par­ents and clinicians need more valid information to guide them on how to interact with a child during painful procedures related to cancer treatment.
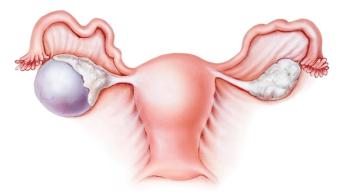
Screening for ovarian cancer in asymptomatic women who are not known to have a high-risk hereditary cancer syndrome has no benefit and may lead to harm due to false positives, according to a new review conducted by the US Preventive Services Task Force (USPSTF).

Patients with nonmetastatic castration-resistant prostate cancer now have their first FDA-approved treatment. Apalutamide (Erleada) was approved based on the phase III SPARTAN trial in which apalutamide reduced the risk of metastasis or death by 72% in patients with nonmetastatic CRPC.

The BD Onclarity HPV Assay can detect up to 14 types of high-risk human papillomavirus (HPV) and identify genotypes that are strongly associated with cervical cancers.

Pfizer announced that the FDA has granted a priority review to a new drug application for lorlatinib, an ALK inhibitor, for use in patients with ALK-positive metastatic non–small cell lung cancer (NSCLC) who have progressed on 1 or more ALK tyrosine kinase inhibitors.

Patient navigation is playing an increasingly vital role in guiding patients with breast cancer through the maze of the American healthcare system. The Patient Navigation Initiative is a pioneering project of Susan G. Komen of Greater New York City focused on helping women overcome barriers to care, which leads to improved outcomes.

Incorporating patient navigation into cancer care makes good business sense. This is the second in a series of articles about of patient navigation.

Eighty percent of hospitalized patients in the U.S. have access to palliative care services. But that means that 20% do not. For patients with cancer, this can potentially mean the difference between effective symptom management and end-of-life care, and inadequate usual care.

Empathy and cultural sensitivity enable navigators to be effective champions for patients with cancer. This is the third in a series of articles about patient navigation.

The LivingWith app helps patients connect with a close circle of contacts and enables them to ask for help with daily tasks, track their symptoms, and collect important documents and information from appointments.
William C. Huang, MD, discusses the transition from open cystectomy to minimally invasive robotic-assisted surgery in the management of muscle-invasive bladder cancer.

Based on findings from the phase III LATITUDE trial, the Food and Drug Adminstration has approved abiraterone acetate (Zytiga) for use in high-risk patients with castration-sensitive prostate cancer.

To ensure the adequate availability of IV solutions that may be in high demand during the flu season, the Food and Drug Administration (FDA) extended the expiration dates of some IV products after Baxter, a major IV solution manufacturer, submitted safety data that indicated that extended expiration dates meet FDA safety standards.

The Canines and Childhood Cancer Study – the first and largest randomized, controlled clinical trial to measure the effects of animal-assisted therapy in the field of pediatric oncology – recently showed significant benefits to families in a time of great need.

Patients whose cancers have metastasized to the brain or central nervous system are often excluded from clinical trials, but now, new guidelines may help researchers identify appropriate clinical trials in which these people may be included.

According to results from an analysis of the Surveillance, Epidemiology, and End Results database of the National Cancer Institute, older adults with colorectal cancer are more likely to develop cardiovascular disease and congestive heart failure.

Changes in cognitive function, or chemobrain, are common among patients who survive breast cancer. However, limited efforts have been put in to understanding or managing these cognitive changes in survivors. Now, there is a web site that can help with just that.

Exercise plays an important role in lymphoma survivorship, a recent study shows.
Researchers have recently discovered that immunotherapy agents have potential to effectively treat desmoplastic melanoma, even though the dense tissue associated with this rare cancer would seem to preclude it.

Immunotherapy alone has not shown much success in treating ovarian cancer, despite success with other cancers, so researchers are now testing combinations of immunotherapy drugs with other agents to see if it enhances effectiveness.
Some underprivileged and minority groups are underrepresented in lung cancer clinical trials, which can affect how and whether they receive appropriate care.

The first large, multicenter trial to investigate the effect of acupuncture in treating aromatase inhibitor-induced joint symptoms in women with breast cancer reported a statistically significant reduction in pain with acupuncture use.
Trastuzumab (Herceptin) did not reduce cardiac function in women with node-positive, HER2-positive, early-stage breast cancer, according a study published in the Journal of Clinical Oncology.

Based on impressive results from the phase III NETTER-1 trial, the FDA has approved Lutathera (lutetium [177Lu] oxodotreotide) for the treatment of patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).

The common skin cream fluorouacil 5%, when applied for 2 to 4 weeks, has been shown to reduce the risk for squamous cell carcinoma for up to a year, according to a study published in JAMA Dermatology.