
Extending anastrozole treatment to 10 years in postmenopausal women with hormone receptor–positive breast cancer who have no evidence of disease after 5 years of standard-of-care treatments.

Extending anastrozole treatment to 10 years in postmenopausal women with hormone receptor–positive breast cancer who have no evidence of disease after 5 years of standard-of-care treatments.

Enzalutamide plus leuprolide lowered the risk of metastases or death by 58% compared with placebo plus leuprolide.

Data presented during the ONS Annual Congress offers guidance for nurses caring for patients receiving darolutamide, docetaxel, and androgen deprivation therapy.

Managing ocular-related adverse effects with mirvetuximab soravtansine is key to helping patients with ovarian cancer stay on treatment.

An analysis of the cilta-cel safety profile yielded promising results, according to nurse investigators.

Milademetan, a MDM2 inhibitor, is associated with thrombocytopenia. An intermittent dosing schedule may help mitigate that adverse event.

Beth Faiman, PhD, MSN, APRN-BC, AOCN, discusses the growing need for advanced practitioner provider education and her role with the NP/PA center of excellence.
Patients who received pembrolizumab plus cisplatin and gemcitabine achieved a median overall survival of 12.7 months, compared with 10.9 months with cisplatin and gemcitabine alone.
Sarah Yenser Wood, RN, MSN, ANP, AOCNP, leads a nursing panel on adverse event management in renal cell carcinoma.
The pathologic complete response rate with durvalumab was 17.2% vs 4.3% with placebo, reflecting an absolute difference of 12.9%.
Early coordination with multiple specialty teams can maximize outcomes and improve quality of life for patients with complex metastatic disease.

Meghan K. Berkenstock, MD, discusses the growing need for strong collaboration between ophthalmologists and gynecologic oncology care teams.
Patients with unresectable pancreatic cancers and cholangiocarcinoma face poor prognoses, but systemic treatment continues to evolve.

With a follow-up of 11.7 months, the estimated 12-month progression-free survival rate was 81% in patients who received neoadjuvant olaparib for BRCA-positive ovarian cancer.
At a median follow-up of 33.3 months, the median overall survival was not evaluable with cemiplimab, vs 20.7 months with chemotherapy alone, in this patient subset.

In a subset of patients with KRAS G12C–mutated non–small cell lung cancer, adagrasib yielded an overall response rate of 68%.

Triple-negative breast cancer often occurs in women younger than 40.

Zev A. Wainberg, MD, discusses the implications of the findings from the phase 3 NAPOLI 3 trial for patients with metastatic pancreatic ductal adenocarcinoma.

As the therapy continues to evolve, best nursing practices become more nuanced.

Patients with head and neck squamous cell carcinoma continued to displayed a survival benefit with pembrolizumab at a 4-year follow-up.

The FDA has approved sacituzumab govitecan-hziy for patients with unresectable locally advanced or metastatic hormone receptor-positive, HER2-negative breast cancer.

First-line ibrutinib plus prednisone missed the primary endpoint in the phase 3 iNTEGRATE trial by not improving the response rate in patients with chronic graft-versus-host-disease compared with placebo plus prednisone.

Nilesh Kalariya, PhD, AGPCNP-BC, AOCNP; and Laura J. Zitella, MS, RN, ACNP-BC, AOCN, discuss practice-changing presentations from the 64th American Society of Hematology Annual Meeting and Exposition.
Encorafenib plus cetuximab, along with chemotherapy, was linked to antitumor activity and a manageable safety profile in patients with BRAF V600E-mutant metastatic colorectal cancer.
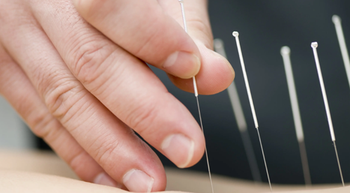
Heather Jackson, PhD, FNP-BC, NEA-BC, FAANP, provides a case-based perspective on the benefit of auricular acupuncture as a tool for managing cancer pain.
Findings from the phase 3 SUNLIGHT study showed that adding bevacizumab to trifluridine/tipiracil boosted overall survival in metastatic colorectal cancer.

The median overall survival with frontline liposomal irinotecan/NALIRIFOX was 11.1 months vs 9.2 months with nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma.
The median overall survival with nivolumab plus chemotherapy was 12.8 months, vs 10.7 months with chemotherapy alone, in patients with treatment-naïve advanced esophageal squamous cell carcinoma.
For patients with CLDN18.2-positive, HER2-negative locally advanced unresectable or metastatic gastric/gastroesophageal junction adenocarcinoma, treatment with zolbetuximab/mFOLFOX6 yielded a progression-free survival (PFS) of 10.61 months whereas placebo/mFOLFOX6 resulted in a PFS of 8.67 months.

In an all-randomized population of patients with advanced gastric cancer, gastroesophageal junction, or esophageal adenocarcinoma, adding nivolumab to chemotherapy yielded a 21% reduction in the risk of death.