
Investigators are evaluating whether eryaspase (Graspa), an L-asparaginase-based therapy that triggers tumor cell death, can extend survival for patients with pancreatic cancer.

Investigators are evaluating whether eryaspase (Graspa), an L-asparaginase-based therapy that triggers tumor cell death, can extend survival for patients with pancreatic cancer.

Investigators are assessing whether Oraxol, an oral formulation of paclitaxel, will be safer and more efficacious than the widely used chemotherapy drug administered by traditional intravenous (IV) infusion.

Investigators are hopeful that idasanutlin, a novel small molecule that targets the MDM2 protein, is being tested in combination with cytarabine in the international phase III MIRROS clinical trial (NCT02545283) to see if omprove the efficacy of chemotherapy in patients with relapsed/refractory acute myeloid leukemia (AML).

The value of axicabtagene ciloleucel (axi-cel; Yescarta), an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in treating R/R MCL will be tested in the multi­center phase II ZUMA-2 clinical trial (NCT02601313).

The FDA has granted the PARP inhibitor rucaparib (Rubraca) a breakthrough therapy designation for single-agent use in adult patients with BRCA1/2-positive metastatic castration-resistant prostate cancer (mCRPC) following at least 1 androgen receptor–directed therapy and taxane-based chemotherapy.

A novel glutaminase inhibitor, CB-839, may provide a survival benefit in combination with cabozantinib (Cabometyx) by cutting off the energy supply to tumor cells in patients with metastatic renal cell carcinoma (RCC).

The phase I open-label LIBRETTO-001 clinical trial is currently testing LOXO-292, an oral small molecule inhibitor of RET signaling, to improve outcomes in patients with RET fusion–positive non–small cell lung cancer (NSCLC), medullary thyroid cancer (MTC), and other tumors with increased RET activity.

Investigators are hopeful that CMB305, a vaccine that boosts the immune response to tumor cells expressing the antigen NY-ESO-1, can improve survival outcomes for patients with synovial sarcoma.

Two randomized phase III clinical trials are designed to determine whether guadecitabine (SGI-110), a potent second-generation hypomethylating agent, could answer unmet medical needs for patients with certain types of blood cancer.

The phase IIb UNITY-NHL clinical trial (NCT02793583) aims to determine whether the novel drug umbralisib (TGR-1202) could be used as monotherapy or in combination with other drugs to treat patients with relapsed/refractory non-Hodgkin lymphoma (NHL).

Selinexor (KPT-330), a promising drug that retards cell malignancy by blocking the transport of tumor-suppressor proteins out of the cell nucleus, is under investigation as combination therapy in heavily pretreated patients with relapsed/refractory multiple myeloma.

A novel inhibitor that targets the AKT node in the PI3K pathway may offer a treatment option for patients with triple-negative breast cancer (TNBC) who are resistant to chemotherapy.

Clinical Trial in Progress: Investigators are seeking to determine whether the combination of eflornithine with lomustine can improve survival for patients with recurrent anaplastic astrocytoma, a rare form of brain tumor.
The combination of eflornithine (alphadifluoromethylornithine) and lomustine is currently being explored as a treatment for patients with recurrent anaplastic astrocytoma (AA).

PEGPH20 (pegvorhyaluronidase alfa), a novel formulation of a naturally occurring enzyme, is being investigated as a biomarker-driven treatment for patients with advanced pancreatic cancer.

Although it is estimated that malignant mesothelioma represents less than 1% of all cancers, it is a fatal asbestos-associated malignancy, and patients with MPM tend to be difficult to treat.

Copanlisib (BAY 80-6946), a novel PI3K inhibitor, is being combined with standard rituximab (Rituxan)-based regimens in patients with relapsed, indolent non-Hodgkin lymphoma (NHL) in 2 phase III clinical trials that investigators hope will expand treatment options in refractory disease settings, particularly with less toxic alternatives.

The phase III ATLANTIS trial is testing the investigational synthetic analogue lurbinectedin in combination with doxoubicin for use in patients with recurrent small cel lung cancer (SCLC).

The novel combination of a neurokinin-1 (NK-1) receptor antagonist and the 5-HT3 receptor antagonist palonosetron (Aloxi), NEPA (Akynzeo) had non-inferior efficacy compared to a conventional aprepitant regimen for preventing chemotherapy-inducted nausea and vomiting (CINV).

For patients with high-risk melanoma, the best option is effective adjuvant therapy which necessitates clincal trials.


An ongoing phase III trial is evaluating the combination of napabucasin, a stemness inhibitor, and FOLFIRI chemotherapy in patients with metastatic colorectal cancer.

ASCO has updated their guidance on the use of second-line hormonal therapy for chemotherapy-naive patients with castration-resistant prostrate cancer (CRPC).
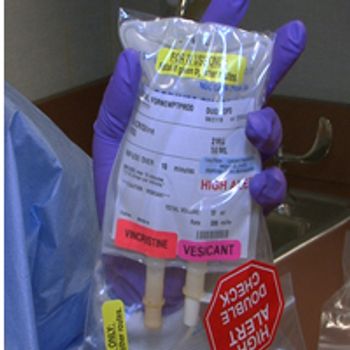
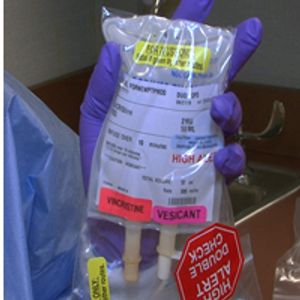
In order to prevent near fatal intrathecal administering of vincristine, the NCCN has launched a campaign "Just Bag It." This campaign pushes for vincristine to be stored in IV drip bags to ensure vincristine is only administered intravenously.
Published: November 16th 2016 | Updated:

Published: May 3rd 2017 | Updated:

Published: July 6th 2017 | Updated:

Published: August 7th 2017 | Updated:
Published: April 4th 2018 | Updated:

Published: October 2nd 2018 | Updated: