
Experts share advice on tailoring frontline treatment and managing toxicities for individuals with hormone receptor–positive metastatic breast cancer.
Bridget Hoyt is the assistant editor for Oncology Nursing News. She earned her BA in communication studies at Rider University with a minor in public relations. She can be reached at bhoyt@mjhlifesciences.com.

Experts share advice on tailoring frontline treatment and managing toxicities for individuals with hormone receptor–positive metastatic breast cancer.

Nurse practitioners give their advice on making treatment choices based on the patient’s medical history and preferences.
The investigational ADC rinatabart sesutecan was given breakthrough therapy designation for use in patients with recurrent/progressive endometrial cancer.

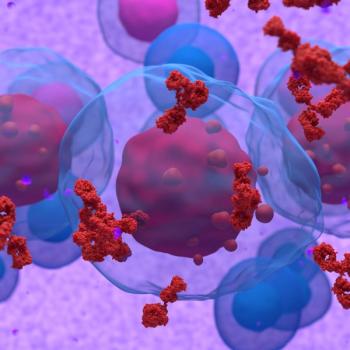
Paolo Tarantino, MD, PhD, discusses ADC structure, toxicity, and nursing consideration for the treatment of patients with breast cancer.

A nurse-led clinic to aid in patient-reported symptom burden had high patient satisfaction.
Neurotoxicities can be monitored long term with personalized questions, according to Mary Steinbach, DNP, APRN.
The NCCN has added zongertinib to its guidelines for the care of patients with HER2 (ERBB2)-mutated non–small cell lung cancer.

Oncology nurses play a key role in monitoring, managing, and personalizing CDK4/6 inhibitor treatment to minimize toxicities and protect quality of life, according to Courtney Moore, APRN, FNP-C, OCN.
Treatment access can still affect patients in urban communities, said Mary Steinbach, DNP, APRN.
A breast cancer survivorship expert shares her top advice for counseling patients on endocrine therapy at the 5-year mark.

A care model led by advanced practice nurses was feasible in providing supportive care and linking providers through post-trial care transitions.

Maria C. Velez, MD, shared that teamwork between pediatric and adult care teams can make the transition of care smoother for AYA patients.
The addition of avutometinib to defactinib showed promising safety and efficacy for patients with low-grade serous ovarian cancer.

Heather Jackson, PhD, FNP-BC, NEA-BC, FAANP, explains how a fellowship program sets the tone for how to transition nurse practitioners to oncology.

FDA accelerated approval was given to zongertinib for the treatment of patients with unresectable/metastatic nonsquamous NSCLC with HER2 TKD activating mutations.

The FDA has granted accelerated approval to dordaviprone for use in adult and pediatric patients with H3 K27M-mutated diffuse midline glioma.
Hope S. Rugo, MD, FASCO, outlined the top considerations for nurses managing toxicities related to PI3K and AKT inhibitors in patients with breast cancer.
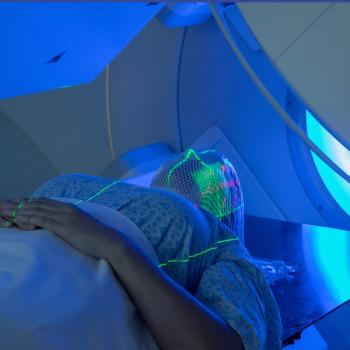
Nurses should be familiar with PET scan protocol and what to ask patients with lung and bone cancers before administering biology-guided radiation therapy.

New FDA approvals in July include therapies for NSCLC, relapsed multiple myeloma, liver cancer, and B-cell malignancies.

Adding aprepitant to chemotherapy was linked to longer survival in patients with non-luminal breast cancers, especially triple-negative breast cancer.
2025 ICE-T Conference presenters explain what nurses and APPs should take into account as immune cell effector therapies become more widely used.
A generic version of ibrutinib was granted tentative approval by the FDA for use in CLL and SLL with 17p deletion and Waldenström macroglobulinemia.

Based on durable response data, TAR-200 has been given priority review for the treatment of patients with high-risk non–muscle-invasive bladder cancer.
Understanding actionable mutations and educating patients on the safety profiles of targeted therapies are essential for the care of HR+, HER2- mBC.
Step-up dosing with remote monitoring resulted in 47% of patients receiving bispecific antibodies remaining outpatient, even in cases of CRS.

The selective internal radiation therapy SIR-Spheres has been approved by the FDA for use in patients with unresectable hepatocellular carcinoma.

Sunvozertinib has received accelerated approval for use in advanced or metastatic non–small lung cancer harboring EGFR exon 20 insertion mutations.

The FDA has given accelerated approval to the BiTE, linvoseltamab, for use in the fifth line of therapy for relapsed/refractory multiple myeloma.

Oncologic therapies approved in June included indications in genitourinary, lung, hematologic, and head and neck cancers.
Formal communication could help ease the transition of care from specialized myeloma centers to community clinic, according to Diane Moran, RN, MA, EdM.