
The NCCN has added taletrectinib to its recommendations for use in all lines of treatments for non–small cell lung cancer harboring ROS1 mutations.
Bridget Hoyt is the assistant editor for Oncology Nursing News. She earned her BA in communication studies at Rider University with a minor in public relations. She can be reached at bhoyt@mjhlifesciences.com.

The NCCN has added taletrectinib to its recommendations for use in all lines of treatments for non–small cell lung cancer harboring ROS1 mutations.
Subcutaneous daratumumab makes it easier for patients to find appointments that fit with their lifestyles and schedules, according to Gina Fries, PA-C.
Illuccix, a preparation kit for injectable Ga-68, has been approved to select patients for radioligand therapy before taxane chemotherapy.

Dato-DXd was granted accelerated approval for use in adults with EGFR-mutated NSCLC after EGFR-targeted therapy and platinum-based chemotherapy.
Per ODAC and CHMP recommendations, subcutaneous daratumumab may become the first approved treatment for smoldering myeloma. Here’s what nurses should know.

The FDA has approved tafasitamab in combination with lenalidomide and rituximab for adults with relapsed/refractory follicular lymphoma.

The FDA approved a perioperative pembrolizumab regimen in head and neck squamous cell carcinoma, marking the first approval in this cancer type in 6 years.

An oral tablet formulation of zanubrutinib was approved for use in patients with certain lymphomas or leukemia and Waldenström macroglobulinemia.

UGN-102 has received FDA approval for use in patients with low-grade intermediate-risk non-muscle-invasive bladder cancer.

The tyrosine kinase inhibitor taletrectinib has been approved for use in patients with ROS1-positive non-small cell lung cancer.

The FDA approved the first oral treatments for paraganglioma, pheochromocytoma, and anal cancer in May.
The FDA approved darolutamide monotherapy for use in metastatic hormone-sensitive prostate cancer following ARANOTE findings.
Patients with stage III resected colon cancer had lower risk of death with less inflammatory diets and more regular physical activity.
The oral TKI sevabertinib has been granted priority review for use in patients harboring HER2 mutations in non-small cell lung cancer.
An oncology nurse shares advice for avoiding exhaustion and soreness when administering subcutaneous injections.
Selecting the right treatment path for a patient with an ESR1 mutation in metastatic breast cancer can help build trust.

The number of CDK4/6-targeting treatment options available for patients with HR-positive breast cancer allows providers to personalize treatment.

Retifanlimab has received approval for the frontline treatment of advanced anal cancer from the FDA.

Telisotuzumab vedotin-tllv has earned accelerated approval for use in patients with non-squamous non-small cell lung cancer with high c-Met overexpression.

Belzutifan has become the first FDA-approved oral therapy for pheochromocytoma or paraganglioma.
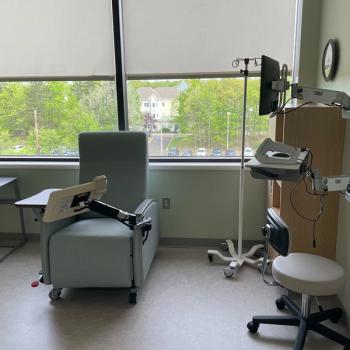
Infusion bays designed with nurse input provide patients the choice between privacy and community while allowing support from loved ones.

The FDA has granted accelerated approval to avutometinib and defactinib in KRAS-mutated recurrent low-grade serous ovarian cancer.

New data show strong overall survival rates, building on previously reported outcomes with efti plus pembrolizumab for head and neck squamous cell carcinoma.

Approvals in oncology during April included treatments for breast cancer, colorectal cancer, and more.
The selective CDK2 inhibitor INX-315 has been given FDA fast track designation for use in CCNE1-amplified, platinum-resistant ovarian cancer.

A ready-to-dilute formulation of thiotepa has been approved by the FDA for use in breast and ovarian cancers.
Both those receiving either expected or less-than-expected starting dosages of ruxolitinib for myelofibrosis treatment reduced daily dosages over 12 months.

The FDA approved penpulimab-kcqx combination and monotherapy for non-keratinizing nasopharyngeal carcinoma.

Emetogenic chemotherapy regimens and back pain were associated with higher symptom burden in older, vs younger, patients with cancer.

When treating patients with hormone-receptor positive, HER2-negative metastatic breast cancer, mutations necessitate the prioritization of quality of life.