
Patients who were given telehealth, vs in-person, exercise programs to manage their symptoms from cancer care experienced similar outcomes, highlighting an alternative for providers to consider.

Patients who were given telehealth, vs in-person, exercise programs to manage their symptoms from cancer care experienced similar outcomes, highlighting an alternative for providers to consider.

Older patients with cancer demonstrated a moderate to high symptom burden, leading to the need for routine symptom assessments and tailored management interventions.

With the progress made with ADCs, there is interest in moving these agents up in the treatment paradigm for HR-positive/HER2-negative breast cancer.
Patients with high-risk prostate cancer treated with a higher dose of radiation therapy and long-term androgen deprivation therapy improved progression-free, cancer-specific, and overall survival compared with the standard dose of radiation.

Patients with advanced hepatocellular carcinoma treated with frontline pembrolizumab plus lenvatinib showed a 3-year or more response among 35% of responders, although additional efficacy results from the trial are consistent with previous findings from the phase 3 LEAP-002 trial.

At a median 39 months of follow-up, zanubrutinib reduced the risk of disease progression by 32% compared with ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma.

Acalabrutinib, whether as monotherapy or as part of a combination, demonstrated superior progression-free survival outcomes compared to obinutuzumab and chlorambucil.

Patients with multiple myeloma are living longer; therefore, their lifelong treatment expenses can become burdensome.

The median progression-free survival was not reached with atezolizumab plus chemotherapy vs 6.9 months in the placebo group of patients with advanced or recurrent endometrial cancer.

Patients with metastatic hormone receptor-positive breast cancer treated with datopotamab deruxtecan experienced a statistically significant and clinically meaningful improvement in progression-free survival.

Treatment with neoadjuvant nivolumab, followed by adjuvant nivolumab after surgery, led to significantly improved event-free survival in the first phase 3 perioperative study in patients with resectable non-small cell lung cancer.

Pediatric patients with leukemia in low- and middle-income countries saw improvements in survival outcomes following implementation of the WHO Framework for Action model.
Apalutamide plus androgen deprivation therapy (ADT) yielded a progression-free survival of 24.5 months vs 21.0 months with ADT alone.

Nivolumab bested placebo in improving disease-free survival rates in patients with muscle-invasive urothelial carcinoma and muscle-invasive bladder cancer.
Phase 2a trial data suggest that single-dose URO-902 may be an effective, and safe, treatment for women experiencing difficulties with bladder control.

Investigators reported an objective response rate of 43% and a disease control rate of 80% among patients with previously treated KRAS G12C–mutated non–small cell lung cancer who received the KRAS G12C inhibitor adagrasib.
Patients with KRAS wild-type pancreatic cancer elicited a significant survival benefit with the addition of nimotuzumab to gemcitabine vs placebo plus gemcitabine in a phase 3 trial.

Combining ibrutinib with rituximab resulted in improved 2-year progression-free survival among elderly patients with previously untreated diffuse large B-cell lymphoma.
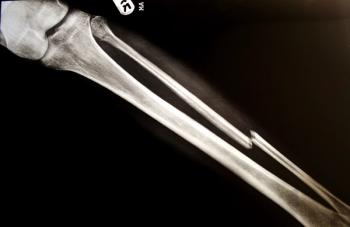
Men with asymptomatic or mildly symptomatic metastatic castration-resistant prostate cancer who are being treated with radium-223 (Xofigo) plus enzalutamide (Xtandi) can reduce their risk of fractures with the use of bone-protecting agents.

The second-generation 4-1BB bi-specific CAR T-cell therapy was efficacious and safe in treating patients with relapsed/refractory B-cell non-Hodgkin lymphoma.


Oncology Nursing News sat down with Brian Rini, MD, from the Vanderbilt-Ingram Cancer Center (VICC), to discuss results from the phase III TIVO03 trial, and its potential impact on patients with relapsed or refractory renal cell carcinoma.

By integrating a comprehensive geriatric assessment with geriatrician-led management care researchers found an improved quality of life for older adults with cancer.

The FDA approved the combination use of encorafenib and cetuximab for the treatment of adult patients with BRAFV600E-positive metastatic colorectal cancer.

The agency granted full approval to pemetrexed for injection (Pemfexy), a liquid injection and branded alternative to Alimta, for nonsquamous NSCLC.

The agency granted the immunoconjugate targeting B-cell maturation antigen priority review to belantamab mafodotin for the treatment of heavily pre-treated patients with relapsed or refractory multiple myeloma.

Oncoprex in combination with osimertinib received fast track designation from the FDA to treat patients with non-small cell lung cancer.

The FDA granted priority review to rucaparib for the treatment of adult patients with BRCA1/2-mutant recurrent, metastatic castration-resistant prostate cancer.

The FDA accepted and granted priority review to the supplemental biologics license application for nivolumab in combination with ipilimumab for the first-line treatment of patients with metastatic or recurrent non-small cell lung cancer.
Trastuzumab deruxtecan had an ORR rate of nearly 61% for patients with advanced HER2-positive breast cancer.