Leukemia
Latest News
Latest Videos
More News

Treatment with acalabrutinib plus venetoclax, with or without obinutuzumab, improved PFS over standard-of-care chemoimmunotherapy in patients with treatment-naive CLL.
Treatment with epcoritamab alone demonstrated deep responses in heavily pretreated patients with CLL, according to the expansion and optimization cohorts in the EPCORE CLL-1 trial.

Long-term follow-up showed continued meaningful responses in patients with relapsed or refractory KMT2Ar acute leukemia who were treated with revumenib, with no new safety signals found.
Treatment with bicistronic CD19/CD22-directed CAR T-cell therapy appeared safe and effective with high remission rates among children with relapsed/refractory B-ALL.
Meta: Blinatumomab plus chemotherapy, vs chemotherapy alone, significantly improved DFS rates and was well tolerated in pediatric patients with standard-risk pediatric B-ALL.
The FDA has granted fast track designation to LBS-007 for the treatment of patients with acute myeloid leukemia.

An expert discusses the FDA approval of obecabtagene autoleucel—the only CAR T-cell therapy given via split dosing for patients with ALL.

The FDA approved an oral solution of imatinib for multiple different cancer types.

The FDA approved an updated drug label for fludarabine phosphate for the treatment of B-cell chronic lymphocytic leukemia.

The FDA approved ninlotinib tablets that do not have mealtime restrictions for Ph-positive CML in chronic phase and CML that was previously treated.

The FDA approved obecabtagene autoleucel (obe-cel; Aucatzyl) for the treatment of adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia.

Brexu-cel is effective in treating patients with relapsed/refractory B-cell acute lymphoblastic leukemia, real-world data shows.

The FDA expanded the approval of methotrexate to include patients with pediatric acute lymphoblastic leukemia and polyarticular juvenile idiopathic arthritis.

The FDA granted an accelerated approval to asciminib for newly diagnosed Ph+ chronic myeloid leukemia in the chronic phase.

The FDA granted a fast track designation to HC-7366 for adults with relapsed or refractory acute myeloid leukemia.

Asciminib is a promising agent for frontline chronic myeloid leukemia, Jorge Cortes, MD, explained.

Older adults with acute myeloid leukemia treated with anthracyclines tended to live longer, but also had more time spent in the hospital compared with those treated with HMAs.

Pediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia are receiving tisagenlecleucel in earlier lines of therapy.

Minimal residual disease status was linked to progression-free survival in patients with chronic lymphocytic leukemia in the first-line treatment setting and with time-limited therapy.
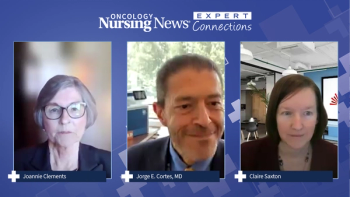
The panel concludes its discussion with insights on challenges and unmet needs in the CML treatment landscape, highlighting ways to better support patients and caregivers.

Experts on chronic myeloid leukemia delve into the important role of informed shared decision-making in the optimal treatment of patients with CML.

Jorge E. Cortes, MD, details clinical scenarios that indicate a need to switch therapies for patients with chronic myeloid leukemia.

Claire Saxton shares insights on how physicians and caregivers can best assist patients with CML who have switched therapies.

A panel of experts on chronic myeloid leukemia discuss common patient concerns, adverse effect considerations, and best practices for setting clear expectations.

Blinatumomab Plus Consolidation Chemo Prolongs Overall Survival in MRD-Negative B-Cell Precursor ALL
Adding blinatumomab to consolidation chemotherapy provided a significant OS benefit in MRD-negative B-cell precursor acute lymphoblastic leukemia.