
Findings from the RATIONALE-306 trial support treatment of tislelizumab plus chemotherapy for PD-L1-positive ESCC.

Findings from the RATIONALE-306 trial support treatment of tislelizumab plus chemotherapy for PD-L1-positive ESCC.

Adding dostarlimab to chemotherapy improved survival vs chemo alone in primary advanced or recurrent endometrial cancer.
Perioperative pembrolizumab plus chemotherapy improves overall survival in patients with resectable gastric cancer.

The monitoring period after CAR T-cell therapy may be safe if shortened due to the lower incidence of ICANS and CRS 2 weeks after treatment in patients with DLBCL.
Oncology nurses can utilize risk assessment to predict patients at increased risk for cisplatin-induced acute kidney injury.

Durable complete responses were observed in patients with TP52-mutated mantle cell lymphoma treated with ibrutinib plus venetoclax.

An expert explores the complexities of immunotherapy during pregnancy, highlighting risks to the fetus and the crucial role of oncology nurses in patient education and management.

The fast track status from the FDA is supported by findings from a phase 1 trial in glioblastoma, which demonstrated the overall survival benefit from DOC1021.

Adding brentuximab vedotin to lenalidomide/rituximab results in a stronger overall survival benefit compared with lenalidomide/rituximab alone in relapsed/refractory diffuse large B-cell lymphoma.

Follow-up with cholesterol control and audiological assessments is suggested for cisplatin-treated patients with cancer.

The FDA granted the investigational menin-MLL inhibitor DSP-5336 fast track designation for KMT2A-rearranged/NPM1-mutant acute myeloid leukemia.

The CAR T-cell therapy ADI-270 received fast track designation from the FDA for pretreated advanced clear cell renal cell carcinoma.

This recommendation is an update to the 2020 ASCO guideline on optimal adjuvant chemotherapy and targeted therapy for the treatment of breast cancer.
The overall survival rate in patients with advanced biliary tract cancer treated with durvalumab plus chemotherapy was nearly double the rate of those treated with chemotherapy alone.

The failure to improve PFS and OS with tiragolumab plus atezolizumab and chemo in metastatic nonsquamous NSCLC indicates missing the primary end points of the SKYSCRAPER-06 trial.
Nivolumab plus ipilimumab improved health-related quality of life and reduced symptom burden in patients with microsatellite instability–high/mismatch repair–deficient mCRC.

Oncology nurses can provide patient and provider education about the benefits of stepped palliative care.

Ciltacabtagene autoleucel demonstrated a statistically significant improvement in overall survival for patients with relapsed/refractory multiple myeloma refractory to lenalidomide.

Oncology nurses play a critical role in identifying and managing rare audiovestibular complications associated with immune checkpoint inhibitors to prevent permanent hearing loss and improve patients' quality of life.

This analysis of patients with previously treated advanced clear cell renal cell carcinoma treated with belzutifan is the largest pooled safety dataset for a HIF-2α inhibitor.

Three-year findings from the TRANSFORM trial provide further evidence that liso-cel should be considered as the new standard of care along with other CAR T-cell therapies for patients with primary refractory or relapsed LBCL, an expert said.
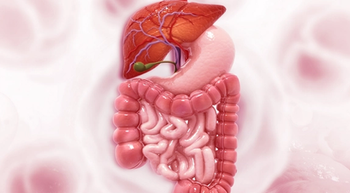
Final results of the ARMANI trial demonstrated that switch maintenance therapy with ramucirumab plus paclitaxel improved survival in advanced HER2-negative gastric or gastroesophageal junction cancer.

Alpha-emitting radiopharmaceutical Radspherin receives a fast track designation from the FDA for peritoneal carcinomatosis from ovarian cancer.

The resubmission of the biologics license application for cosibelimab for locally advanced or metastatic cutaneous squamous cell carcinoma follows a complete response letter from the FDA in December 2023.
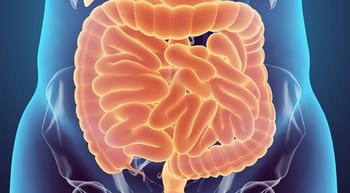
Disease-free survival and overall survival improved with celecoxib plus advanced chemotherapy in patients with PIK3CA-mutated stage III colon cancer.

The clinical practice guideline from the American Society for Radiation Oncology focuses on the use of definitive and postoperative radiation therapy in patients with HPV-associated oropharyngeal squamous cell carcinoma.

With several options available for the second-line treatment of HR-positive, HER2-negative mBC, an expert emphasized the importance of tailoring treatment for every patient.

The updated risk evaluation and mitigation strategies for autologous CAR T-cell therapy removed requirements for training and educational materials about the risk for certain toxicities.
Daniel Verina, DNP, ACNP-BC, discussed how CAR T-cell therapy is changing the treatment landscape for patients with multiple myeloma.

Durvalumab plus chemotherapy in the perioperative setting met event-free survival and overall survival endpoints in MIBC.