
Multiple Initiatives Contribute to ESPY Week Fundraising

An oncology nurse started a grassroots campaign that brought together community members and patients on the oncology unit.

A person’s HIV status should not be the sole reason they are excluded from a cancer clinical trial, according to recent research.

A pediatric oncology nurse’s experience came full circle following her own bladder cancer diagnosis, followed by serving as a caregiver to her husband who faced the same disease 6 years later.
Symptom management and proper risk stratification are two crucial ways to improve the care of patients with myeloproliferative neoplasms, according to one expert.
By better understanding the subtypes of breast cancer, clinicians can create more personalized and effective treatment plans for patients with breast cancer.
After her sister passed away from hepatocellular carcinoma, Andrea Wilson started Blue Faery: The Adrienne Wilson Liver Cancer Association.
When it comes to molecular testing to advance the field of lung cancer, coordinated efforts between providers—and institutions nationwide—are key.

CLR 131, a targeted, molecular radiotherapy, showed a 33% overall response rate in patients with diffuse large B-cell lymphoma.

Now that the CD19-targeted CAR T-cell therapies axicabtagene ciloleucel (axi-cel; Yescarta) and tisagenlecleucel (Kymriah) have shown durable responses in the relapsed/refractory settings of non-Hodgkin lymphoma, researchers are hopeful that earlier exposure may heighten the curative potential of the modality, explained Mazyar Shadman, MD, MPH.
Now that patients have more drug options to treat myeloma, quality of life is becoming more important, said one expert.
An expert discusses the cardiac toxicity of ABP 980, a trastuzumab biosimilar, in the breast cancer space.
An expert gives an overview of how CDK4/6 inhibitors changed the treatment landscape for patients with metastatic breast cancer.

The Food and Drug Administration (FDA) approved selinexor (Xpovio) plus dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma, who had at least 4 prior therapies.

As the number of cancer diagnoses continues to increase across the United States it is crucial that primary care providers know when to refer patients to oncology teams.

Here are the top 5 Oncology Nursing News stories for June 2019.
Switch maintenance treatment with pembrolizumab (Keytruda) improved progression-free survival (PFS) in patients with metastatic urothelial cancer who have stable disease following frontline platinum-based chemotherapy, according to results of a randomized phase II study that were presented at the 2019 ASCO Annual Meeting.

The Food and Drug Administration approved bevacizumab-bvzr (Zirabev) – a biosimilar for bevacizumab (Avastin) for the treatment of metastatic colorectal cancer; unresectable, locally advanced, recurrent or metastatic NSCLC; recurrent glioblastoma; metastatic renal cell carcinoma; and persistent, recurrent or metastatic cervical cancer.

The Food and Drug Administration approved daratumumab (Darzalex) plus lenalidomide and dexamethasone (Rd) for the treatment of patients with newly diagnosed myeloma who are not eligible for autologous stem cell transplant (ASCT), according to Janssen, the manufacturer of the drug.

With a goal of championing the value of patient navigators, Susan G. Komen Greater New York City and NYU Langone have teamed up to provide patients with improved care in Brooklyn through a new patient navigation project.

The Food and Drug Administration lifted a partial clinical hold on the CANOVA trial, examining venetoclax with dexamethasone in relapsed/refractory myeloma.

While clinical trials drive FDA approvals, real-world data may be equally important as it lends insight to a broader group of patients.

The FDA has granted a priority review designation to a supplemental biologics license application for niraparib (Zejula) for the treatment of patients with advanced ovarian cancer.

The FDA has granted an approval to a prefilled syringe for lanreotide (Somatuline Depot), which has been designed to enable healthcare providers to administer the injection easier, for the treatment of adults with unresectable, well or moderately differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors.
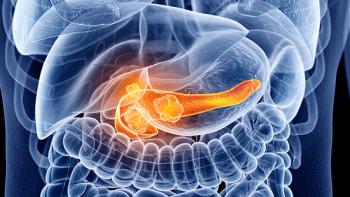
Pancreatic cancer mortality is on the rise compared with other gastrointestinal malignancies, spurring an abundance of novel treatment approaches to combat the disease.
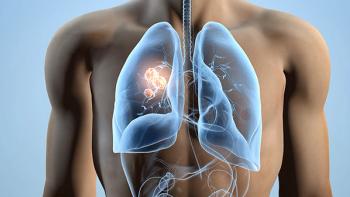
Personalized treatments are drastically improving the outcomes of patients with non-small cell lung cancer, but there is still work to be done when it comes to understanding drug resistance.

Biosimilars continue to push their way into the oncology market. They're different from generic drugs. Here's what nurses should know.
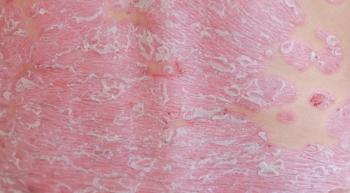
Oncology nurses shouldn’t be concerned if patients with non-small cell lung cancer experience skin toxic effects after immunotherapy.

The FDA approved pembrolizumab (Keytruda) for patients with advanced small cell lung cancer (SCLC) who experienced disease progression after 2 or more prior lines of therapy.

Oncology nursing is more than just a job to Barbara Bittner, RN, OCN.