
The Food and Drug Administration (FDA) approved trastuzumab-anns (Kajinti), a biosimilar of trastuzumab (Herceptin) for the treatment of HER2-overexpressing breast cancer, metastatic gastric or gastroesophageal junction adenocarcinoma.

The Food and Drug Administration (FDA) approved trastuzumab-anns (Kajinti), a biosimilar of trastuzumab (Herceptin) for the treatment of HER2-overexpressing breast cancer, metastatic gastric or gastroesophageal junction adenocarcinoma.

The FDA approved pembrolizumab alone or in combination with 5-FU to treat recurrent or metastatic head and neck squamous cell carcinoma.

The FDA has granted an accelerated approval to polatuzumab vedotin (Polivy) for use in combination with bendamustine and rituximab (Rituxan; BR) for the treatment of patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received at least 2 prior therapies.

The FDA granted a fast track designation to momelotinib for the treatment of patients with intermediate/high-risk myelofibrosis who have previously received a JAK inhibitor.

Here are the top 5 Oncology Nursing News stories for May 2019.
Monoclonal antibodies, proteasome inhibitors, and targeted agents are among the many options in the crowded treatment landscape of relapsed/refractory multiple myeloma, and the emergence of drugs, such as venetoclax (Venclexta) and selinexor, could add to the complexity of this paradigm, said Cristina Gasparetto, MD.

Studies have shown that heated chemotherapy may benefit women with ovarian cancer, but is this the right treatment for everyone? One expert weighs in.

The FDA granted a priority review to daratumumab (Darzalex) in combination with bortezomib (Velcade), thalidomide and dexamethasone (Vtd) for the front-line treatment of newly diagnosed patients with multiple myeloma who are candidates for autologous stem cell transplant.


The results of genetic testing could have major implications for patients with breast cancer and their families.

Healthcare providers should not base cancer care with immune checkpoint inhibitors on gender, according to recent study findings.

Trebek's current outcome could offer a source of hope for patients with pancreatic cancer, who typically have poor prognoses.

The FDA has approved the R2 regimen of lenalidomide (Revlimid) plus rituximab (Rituxan) for use in patients with previously treated follicular lymphoma and marginal zone lymphoma (MZL).
In non–small cell lung cancer (NSCLC), KRAS G12 mutations have been historically difficult to target, and research efforts dedicated to the development of effective targeted therapies for NRG1 fusions have not been successful. However, several biopharmaceutical companies are in the early stages of addressing that challenge, explained Sai-Hong I. Ou, MD, PhD.

Genetic and genomic testing are becoming more popular and useful in the cancer space.
There may be a correlation between androgen deprivation therapy and cardiac complications, but more research is still needed.

The FDA has approved the PI3K inhibitor alpelisib (Piqray) for the treatment of postmenopausal women, and men, with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer following progression on or after an endocrine-based regimen.

The Food and Drug Administration (FDA) approved ruxolitinib (Jakafi) for the treatment of patients 12 years or older with steroid-refractory acute graft-versus-host disease.

May is Skin Cancer Awareness month. What are some of the important conversations nurses should be having with their patients about the disease?

The FDA has approved the NovoTTF-100L System in combination with pemetrexed and platinum-based chemotherapy for the frontline treatment of patients with unresectable, locally advanced or metastatic malignant pleural mesothelioma (MPM), marking the first treatment for this patient population in more than 15 years.

Funds Benefit Cancer Research in N.C. and Beyond

Nurses now have a wealth of new and updated resources to ensure their patients with prostate cancer can make the best treatment choices they can, thanks to the Prostate Cancer

Through her Bravery Bags program, Harding Cranford brings together members in the healthcare setting and the community to brighten patients’ days.
Investigators are working on expanding immunotherapy use for this patient population, and genetic testing will be more important than ever.
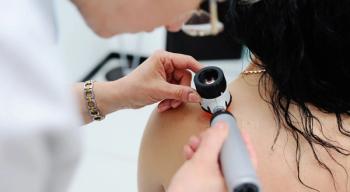
Compared to the general population, survivors of childhood cancer who live 5 or more years from diagnosis are at a higher risk for skin cancer.
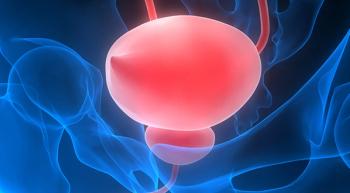
Two new urine tests currently being investigated for bladder cancer detection and monitoring have demonstrated more than 90% sensitivity and specificity – highlighting the potential for addressing an unmet need among this patient population.

This was the first large, randomized clinical trial to show that diet can reduce the risk of dying from breast cancer.

Lenalidomide (Revlimid) significantly reduced the risk for smoldering multiple myeloma to progress to cancer among patients with moderate to high risk, according to findings from the phase II/III E3A06 trial to be presented at the 2019 ASCO Annual Meeting.

A group of researchers in the UK sought out to determine the efficacy of a reduced-dose regimen of oxaliplatin (Eloxatin) and capecitabine (Xeloda) in this patient population through the randomized, phase III GO2 clinical trial.

The FDA has approved the combination of venetoclax (Venclexta) and obinutuzumab (Gazyva) for the frontline treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).