New research found that the discontinuation of prophylactic octreotide was not associated with an increased rate or duration of carcinoid crisis in patients with neuroendocrine tumors.

New research found that the discontinuation of prophylactic octreotide was not associated with an increased rate or duration of carcinoid crisis in patients with neuroendocrine tumors.

The director at the Center for Cancer Health Equity, The University of Ohio Comprehensive Cancer Center, discusses how the pandemic put a spotlight on issues of equity within the health care system.

Trilaciclib was associated with reduced chemotherapy-induced myelosuppression and the need for associated supportive care in patients with a subtype of lung cancer.

As nurses around the country weigh their professional options, some wonder if the answer resides in telling a white lie. But every choice has a consequence. What are the ramifications of lying on a religious exemption form?

Ropeginterferon alfa-2b-njft is now an FDA-approved drug for the treatment of polycythemia vera.

Nuanced and multidisciplinary decision making are needed to optimize and personalize locoregional, systemic, and supportive care in metastatic breast cancer.

An expert from NYU Langone outlines how checkpoint blockade approvals have helped advance endometrial cancer management.

David Hui, MD, MSc, from The University of Texas MD Anderson Cancer Center, discusses the impacts of personal sedation goals for caregivers of patients with cancer experiencing end-of-life agitated delirium.

The COVID-19 pandemic has demonstrated that personalized medicine should consider social determinants of health disparities as well as genomic factors.
Stephanie Jackson, DNP, MSN, RN, AOCNS, BMTCN, Oncology Nursing News co-editor in chief, discusses omacetaxine as a novel regimen in CML treatment.
Patients with cancer should receive the entire COVID-19 vaccination as soon as possible, according to an expert at the 39th Annual Chemotherapy Foundation Symposium.

“Health care providers should be aware of this gap in care for dual-eligible patients and other vulnerable populations so that needs can be identified and resources can be appropriately directed.”

Findings from biomarker tests for patients with chronic lymphocytic leukemia can help appropriately tailor therapy plans as well as identify patients eligible for clinical trials.

In addition to experiencing poor emotional health, a high percentage of nurses have experienced trauma and a desire to leave their position because of the COVID-19 pandemic.

Newer modalities seek to use MRD to provide more definitive prognoses.
A recent study found that nurses demonstrated a higher preference for deep sedation to treat patients with cancer-related delirium, compared with caregivers.

After 6 months of additional follow-up, patients with resected high-risk stage II melanoma continued to experience improved relapse-free survival following treatment with adjuvant pembrolizumab.

More research on the various clinical, pathological, and outcome differences in myoepithelial tumors is needed to offer better prognoses for patients with varying tumor characteristics.

Between 2000 and 2018, the number of pancreatic cancer cases rose, particularly among women between the ages of 15 to 35 years.
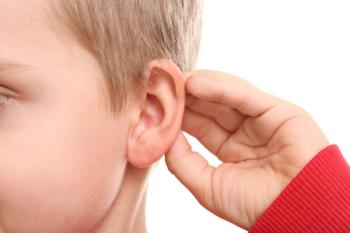
Cisplatin-induced hearing loss was found to be more prevalent in very young children receiving treatment; therefore, findings suggest that audiological monitoring be conducted in a standardized manner for those undergoing this treatment.

The new Cilta-Cel PDUFA decision date is February 28, 2022.
A survey completed by nurses and APPs revealed that many healthcare professionals may be blind to their own implicit biases.
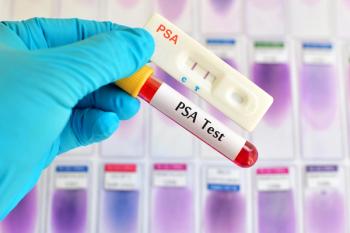
The increased risk of prostate cancer associated with MSH2 and MSH6 pathogenic variants point to a need for annual PSA screening in men over the age of 40.

CAR T-cell therapies represent a potent treatment option for patients with heavily pretreated relapsed/refractory hematologic malignancies, however, the infection risk associated with treatment may hinder the efficacy of COVID-19 vaccines in this population.
A final analysis of the phase 3 ClarIDHy trial revealed that OS was superior in patients receiving ivosidenib, despite a high crossover rate from the placebo group.

Kristin Rupp, RN, OCN, BSN, reminiscences about the experiences that made her the nurse she is today, and how she continues to pay it forward.

Ruxolitinib was not associated with an increased risk of secondary malignancies in patients with polycythemia vera.

Parsaclisib has been granted a priority review for 3 indications that include relapsed or refractory follicular, marginal zone, and mantle cell lymphoma.

“It is not a ‘one and done kind of thing.” It is very important to implement these skills throughout the community. It's a practice. It's a behavioral change.”
An expert from Dana-Farber Cancer Institute comments on ongoing research in translocation RCC and next steps for research in this treatment space.