Hematology
Latest News
Video Series
Latest Videos
Podcasts
CME Content
More News
The addition of navtemadlin to ruxolitinib for patients with myelofibrosis who are JAK inhibitor-naive with suboptimal response to ruxolitinib will be evaluated in the phase 3 POIESIS trial.
Revumenib was added to NCCN guidelines for relapsed or refractory NPM1-mutated acute myeloid leukemia.
Off-the-shelf mosunetuzumab/polatuzumab vedotin produced durable responses with manageable safety in BTK inhibitor–exposed relapsed/refractory MCL.

Read nursing considerations for treating patients with mantle cell lymphoma using lisocabtagene maraleucel.
Mosunetuzumab plus polatuzumab vedotin increased PFS and response rates vs R-GemOx in transplant-ineligible large B-cell lymphoma.
New AQUILA data show PFS improvement in patients with smoldering multiple myeloma who were treated with subcutaneous daratumumab vs active monitoring.
Mortality, progression, and venous thromboembolism were among outcomes improved for patients with polycythemia vera taking a GLP-1a.
Patients with intermediate- or high-risk MDS experienced a higher modified overall response rate with the venetoclax/azacitidine vs placebo/azacitidine.
Biology-guided radiotherapy involves teamwork across multiple specialists, according to Samantha Bianzon, BSN, RN.

Nurses must stay up to date on novel agents and their toxicities to properly monitor for and manage immune effector cell-associated neurotoxicity syndrome.

CRS is a common but manageable toxicity of CAR T-cell therapy and bispecific antibodies. Learn strategies to identify and manage this adverse effect.

Beyond administering CAR T-cell therapy and bispecifics, oncology nurses must apply proactive, supportive care and an understanding of complex treatments.

A nurse-led clinic to aid in patient-reported symptom burden had high patient satisfaction.
Neurotoxicities can be monitored long term with personalized questions, according to Mary Steinbach, DNP, APRN.
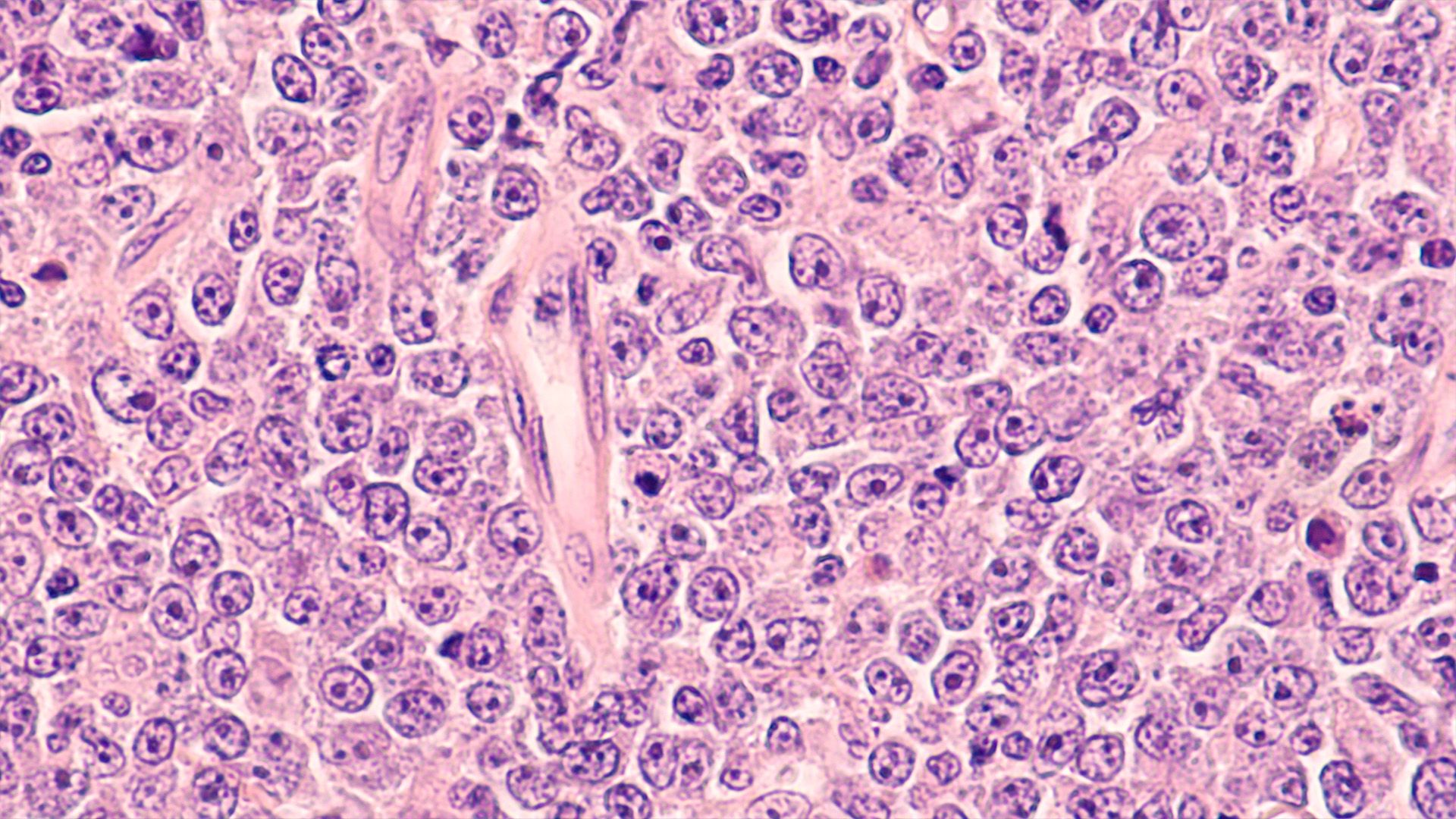
An observational study found IHC may serve as a biomarker for early detection of TP53‐mutant MDS or AML and prediction of TP53 allelic state.
Nurses should be familiar with PET scan protocol and what to ask patients with lung and bone cancers before administering biology-guided radiation therapy.

New FDA approvals in July include therapies for NSCLC, relapsed multiple myeloma, liver cancer, and B-cell malignancies.
Adding sonrotoclax to zanubrutinib led to deep and durable response in relapsed or refractory mantle cell lymphoma, according to EHA Congress data.
2025 ICE-T Conference presenters explain what nurses and APPs should take into account as immune cell effector therapies become more widely used.
A generic version of ibrutinib was granted tentative approval by the FDA for use in CLL and SLL with 17p deletion and Waldenström macroglobulinemia.

Oncology nurses can assess patients’ risk factors and advocate for preventive strategies that protect kidney function during cisplatin therapy.

The FDA removed REMS and reduced certain monitoring needs for liso-cel and ide-cel in B-cell malignancies.
The frontline combination of ibrutinib with venetoclax was associated with complete response and durable remission for older patients with MCL.
Per ODAC and CHMP recommendations, subcutaneous daratumumab may become the first approved treatment for smoldering myeloma. Here’s what nurses should know.