Immunotherapy agents may soon combine with VEGF TKIs to redefine the frontline standard for treatment of metastatic renal cell carcinoma (mRCC).
Caroline Seymour joined MJH Life Sciences in 2018 and has expertise in video production and print/digital publication. Email: cseymour@onclive.com

Immunotherapy agents may soon combine with VEGF TKIs to redefine the frontline standard for treatment of metastatic renal cell carcinoma (mRCC).

The presence of HPV-16 and -18 were associated with a higher risk of developing high-grade cervical intraepithelial neoplasia (CIN) in women under the age of 30, study says.
Low NR2F1 expression in disseminated tumor cells (DTCs) that is found in bone marrow may be indicative of developing metastatic breast cancer, according to results of a study published in Breast Cancer Research.
Findings from the MONALEESA-3 trial dispelled the theory that a CDK4/6 inhibitor had to be reserved following recurrence on hormone therapy in postmenopausal patients with hormone receptor (HR)-positive, HER2-negative breast cancer, explained Dennis J. Slamon, MD, PhD.
While revisions to guidelines have given clinicians a better handle on how to manage immune-related adverse events (AEs), they have not eliminated the need for individualized treatment of patients with genitourinary (GU) cancers, says Xiao X. Wei, MD, MAS.
Over the last few years, treatments for patients with metastatic hormone receptor (HR)-positive breast cancer have evolved rapidly. As changes in care continue to progress, nurses need to understand these treatment options, and where the research may take the field in the future.
Adverse effects from immunotherapy for genitourinary cancer typically require a multidisciplinary approach among health care teams.
With the approval of rucaparib in April 2018, there are now 3 PARP inhibitors approved for use in the maintenance setting for patients with ovarian cancer who are in a complete or partial response to platinum-based chemotherapy, and they are significantly improving progression-free survival.
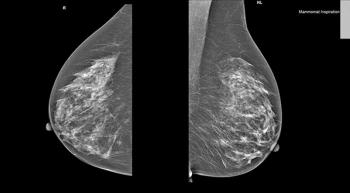
The National Comprehensive Cancer Network (NCCN) guidelines on breast cancer now allow physicians to pinpoint the women who are most likely to benefit from post-mastectomy radiation using the latest targeted testing techniques.
The surgical landscape for non-small cell lung cancer is shifting now that durvalumab (Imfinzi), the first immunotherapy for stage III NSCLC, has been approved for the FDA.
William C. Huang, MD, discusses the transition from open cystectomy to minimally invasive robotic-assisted surgery in the management of muscle-invasive bladder cancer.