
Accelerated approval was granted by the FDA to ponatinib plus chemo for adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia.

Accelerated approval was granted by the FDA to ponatinib plus chemo for adult patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia.

The Oncologic Drugs Advisory Committee of the FDA unanimously voted in favor of ciltacabtagene autoleucel for the treatment of early relapsed/refractory myeloma.

The FDA granted an orphan drug designation to avutometinib either alone or in combination with defactinib for recurrent low-grade serous ovarian cancer.

The benefits of imetelstat outweigh the risks for anemia treatment in lower-risk myelodysplastic syndrome, according to a vote by the FDA’s Oncologic Drugs Advisory Committee.

Accelerated approval has been granted by the FDA to lisocabtagene maraleucel for the treatment of certain patients with relapsed/refractory CLL or SLL.

Tislelizumab-jsgr (Tevimbra) has been approved for unresectable or metastatic esophageal squamous cell carcinoma (ESCC) among adult patients who have previously received systemic chemotherapy that did not include a PD-1/PD-L1 inhibitor.

In a 16-to-2 vote, the FDA's Medical Imaging Drugs Advisory Committee voted in support of the benefit-risk profile of Lumisight to detect cancerous tissue during breast conservation surgery.

A biologics license application has been accepted by the FDA for first-line tislelizumab plus chemotherapy for gastric/gastroesophageal junction cancer.

The FDA approved zanubrutinib plus obinutuzumab for the treatment of patients with relapsed or refractory follicular lymphoma after receiving 2 or more lines of systemic therapy.

Nivolumab plus cisplatin and gemcitabine received FDA approval for the first-line treatment of adult patients with unresectable or metastatic urothelial carcinoma.

The antibody-drug conjugate inotuzumab ozogamicin was approved by the FDA for the treatment of patients aged 1 year and older with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia.

An orphan drug designation has been granted by the FDA to ocifisertib as a potential treatment option in acute myeloid leukemia.

Priority review was granted by the FDA to a supplemental new drug application for adagrasib plus cetuximab for the treatment of advanced KRAS G12C–mutated colorectal cancer.

Amivantamab was approved by the FDA as first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations or as therapy for those whose disease progressed on or after platinum-based chemotherapy.

The label expansion for ibrutinib with an oral suspension formulation has been approved by the FDA in all current indications including Waldenström macroglobulinemia, chronic lymphocytic leukemia/small lymphocytic lymphoma, and chronic graft-versus-host disease.

A supplemental Biologics License Application for teclistamab-cqyv (Tecvayli) has been approved by the FDA at a reduced dose of 1.5 mg/kg every 2 weeks for the treatment of patients with relapsed/refractory multiple myeloma who have maintained a complete response or greater for at least 6 months.

The first real-time, non-invasive skin cancer evaluation system has received clearance from the FDA.

The FDA approved osimertinib for use with platinum-based chemotherapy to treat patients with locally advanced or metastatic non-small cell lung cancer that harbor EGFR exon 19 deletions or exon 21 L858R mutations.

Lifileucel is the first TIL therapy approved by the FDA for a solid tumor, specifically previously treated unresectable or metastatic melanoma.

The FDA granted an orphan drug designation to soquelitinib for potential use in T-cell lymphoma.

Tepotinib has received a traditional approval from the FDA for metastatic NSCLC with MET exon 14 skipping alterations, updating the previous accelerated approval from 2021.

Irinotecan liposome has been approved by the FDA for use with oxaliplatin, leucovorin, and fluorouracil to treat patients with metastatic pancreatic adenocarcinoma.

Fast track designation has been granted by the FDA to vepdegestrant for the treatment of select patients with ER-positive/HER2-negative locally advanced or metastatic breast cancer.
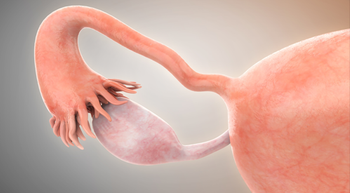
FDA granted a fast-track designation to the TROP2-directed ADC BNT325/DB-1305 for the treatment of platinum-resistant ovarian epithelial, fallopian tube, or primary peritoneal cancer.
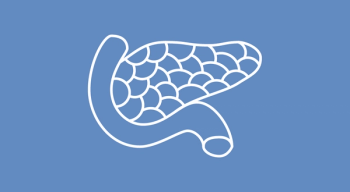
The FDA granted efineptakin alfa an orphan drug designation, deeming it a potential treatment option for pancreatic cancer.

A priority review has been granted by the FDA to trastuzumab deruxtecan for the treatment of unresectable or metastatic HER2-positive advanced solid tumors.

Erdafitinib was approved for the treatment of adults with locally advanced or metastatic urothelial carcinoma, which amends a previously approved indication of the drug.

Pembrolizumab plus chemoradiotherapy has been approved by the FDA for the treatment of patients with FIGO 2014 stage III to IVA cervical cancer.

A supplemental biologics license application has been granted priority review by the FDA, which seeks a full approval of tisotumab vedotin for patients with recurrent or metastatic cervical cancer that progressed on or following frontline therapy.

The Food and Drug Administration approved an on-body delivery system for pegfilgrastim-cbqv, a biosimilar of pegfilgrastim.