
An expert discusses 2 new surgical treatments for breast cancer-associated lymphedema.

With the approval of 2 new JAK inhibitors for the management and treatment of myelofibrosis, the space is undergoing a new progression, with lots of questions left to answer.

A new study shows promising results for niraparib in the treatment of patients with ovarian cancer.

Sometimes, there's only so much you can do for patients in a clinical setting. But by sharing their stories and struggles, you can drive change that will affect their clinical treatment.
At the 2019 World Conference on Lung Cancer, an NSCLC expert weighed in on the usefulness of cell-free DNA-based testing versus traditional tissue testing for genomic sequencing.


The FDA has granted accelerated approval to the combination of lenvatinib and pembrolizumab in the treatment of patients with advanced endometrial carcinoma.

In 2019, 18,000 patients will die from metastatic urothelial cancer, but a new priority review recently granted to enfotumab vedotin shows promise for future treatment of patients with the disease.

In this episode of CURE Talks Cancer Podcast: Nursing Edition we speak with an oncology nursing expert on a topic not addressed enough, workplace violence.

Throughout all of the cancer landscape, there are racial disparities in the way patients are treated, and for mesothelioma patients, those disparities can make the difference between life and death.

Treating patients with HER2-positive metastatic breast cancer is difficult, and patients can fail multiple lines of direct treatment. But now, the FDA has accepted a supplemental new drug application for a combination treatment to help these patients failing multiple treatments.

In about 3% to 5% of patients with NSCLC MET alterations are presented that can drastically change a patient's prognosis. Now, an investigational MET inhibitor called tepotinib was granted a breakthrough designation by the FDA, and it may prove beneficial for patients in this space.
Targeted therapies for patients with EGFR-mutated and HER2-positive NSCLC have yet to see widespread success, but these 3 novel therapies, presented at the 2019 World Conference on Lung Cancer, show promise for this patient population.

The FDA has granted a fast track designation to the KRAS inhibitor AMG 510 for the treatment of patients who received prior treatment with KRAS G12C-mutated NSCLC.
Treating patients with multiple myeloma can be difficult, but in the past several months there have been a large number of promising updates to potential new combinations of treatment.

Patients with METex14-Mutated NSCLC potentially have a new first-line treatment to look towards as Capmatinib has been granted a breakthrough designation by the FDA.

One expert discusses how immunotherapy is changing the treatment – and adverse event – landscape in oncology.
When one of your patients is diagnosed with gastrointestinal cancer, it can be difficult for them to cope with their diagnosis. However, as their Oncology nurse, you can help guide them to reliable patient resources on stomach cancer, like Debbie’s Dream Foundation.

Here are the top 5 Oncology Nursing News stories for August 2019.

A certified genetic counselor discusses the role of genetic testing in gastrointestinal cancers.

Patients are getting a ton of information from outside sources and advertisements about new immunotherapy agents. It is crucial that providers offer them the key facts that they need.

This week, Johnson & Johnson was ordered to pay $572 million for contributing to the opioid crisis in the state of Oklahoma.

The frontline combination of nivolumab (Opdivo) and ipilimumab (Yervoy) showed a robust and durable clinical benefit in patients with metastatic colorectal cancer (mCRC) whose tumors are microsatellite instability–high (MSI-H)/mismatch repair deficient (dMMR)—a population with a historically poor prognosis, said Heinz-Josef Lenz, MD, FACP.
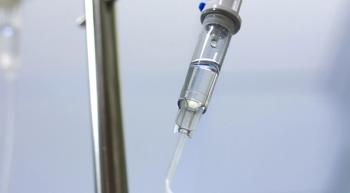
Before considering intraperitoneal chemotherapy, or dose-dense chemotherapy, for newly diagnosed patients with advanced ovarian cancer, it's not vital to first determine whether the patient is medically fit for these treatments.

Chemotherapy is the foundation of treatment for patients with recurrent ovarian cancer, but according to Madeleine B. Courtney Brooks, MD, MPH, conversations regarding the potential for secondary debulking and use of bevacizumab (Avastin) may also be warranted.

While the US Preventative Services Task Force expanded their recommendations of who should be tested for BRCA mutations, there are still issues left unaddressed, according to one expert.

The FDA has granted a priority review designation to a supplemental new drug application for enzalutamide (Xtandi) for the treatment of men with metastatic hormone-sensitive prostate cancer (mHSPC), according to Astellas Pharma Inc. and Pfizer Inc., the codevelopers of the androgen receptor inhibitor.

There are multiple steps nurses must take to prevent and manage pulmonary toxicities in patients taking immunotherapy.

Two different randomized clinical trials not only validated the value of annual lung cancer screening in patients, but also can open the doors for vital smoking cessation talks.

The Centers for Medicare and Medicaid Services (CMS) announced that it will cover chimeric antigen receptor (CAR) T-cell therapy for Medicare beneficiaries.