
The Bonnie J. Addario Lung Cancer Foundation (ALCF) is partnering with ALK Positive, an online community of over 1,200 members worldwide dealing with ALK-positive lung cancer, to encourage Lung Cancer Registry membership.

The Bonnie J. Addario Lung Cancer Foundation (ALCF) is partnering with ALK Positive, an online community of over 1,200 members worldwide dealing with ALK-positive lung cancer, to encourage Lung Cancer Registry membership.
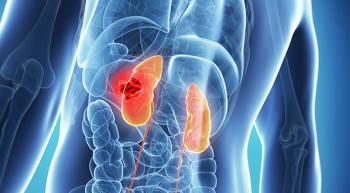
Combining the PD-L1 inhibitor avelumab (Bavencio) with the VEGF inhibitor axitinib (Inlyta) significantly improved progression-free survival (PFS) compared with sunitinib (Sutent) in treatment-naïve patients with advanced renal cell carcinoma (RCC), according to findings from the phase III JAVELIN Renal 101 study.

2018 Hurricane preparedness fund to aid Southeastern residents living with cancer

A study published in Blood demonstrated the significant disparities found between insurance status and survival outcomes in patients of all ages with follicular lymphoma.

An expert discusses tips on how patients with cancer can manage their "scanxiety."

Women aged 65 and older undergoing chemotherapy for breast cancer have a higher risk of experiencing a decline in their ability to function physically, according to a new study published in the Journal of the American Geriatrics Society.
While revisions to guidelines have given clinicians a better handle on how to manage immune-related adverse events (AEs), they have not eliminated the need for individualized treatment of patients with genitourinary (GU) cancers, says Xiao X. Wei, MD, MAS.

Patients with multiple myeloma who are older than 75 years could be viable candidates for autologous stem cell transplantation (ASCT).
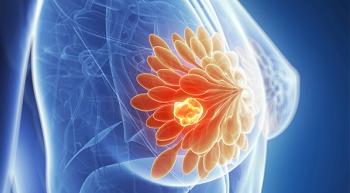
Banu K. Arun, MD, discussed the challenge of treating patients with triple-negative breast cancer, the value of genotyping, and what she hopes to see in future trial results.

A new tool could change the surgical landscape of ovarian cancer treatment.

CURE Media Group’s flagship product, CURE® magazine, the nation’s leading consumer digital and print media enterprise focused on patients with cancer, will host its monthly Tweet Chat on Thursday, September 27, at 1 p.m. EDT, for readers to ask questions and to facilitate discussions about women’s cancers.

The FDA has approved the CD22-directed recombinant immunotoxin moxetumomab pasudotox (Lumoxiti) for the treatment of adult patients with relapsed or refractory hairy cell leukemia (HCL) who have received at least 2 prior lines of therapy, including treatment with a purine nucleoside analog.

A study evaluating the implementation of a multifaceted approach to bedside handoffs incorporating the teach-back method and discharge bundles on an inpatient oncology unit at a large military treatment facility showed that patient education reduced patient readmission rate.

The Food and Drug Administration (FDA) granted priority review to pembrolizumab (Keytruda) monotherapy for the first-line treatment of patients with locally advanced or metastatic nonsquamous or squamous non-small cell lung cancer (NSCLC) whose tumors express PD-L1 without EGFR or ALK genomic tumor aberrations.

Researchers conducted a study to determine if virtual reality is effective in reducing anxiety and pain prior to and during a bone marrow aspiration and biopsy.

Ad to ask people to share how they are more than a patient on social media

The FDA granted breakthrough therapy designation to the oral agent LOXO-292 for the treatment of RET fusion–positive non–small cell lung cancer (NSCLC) or RET-mutant medullary thyroid cancer (MTC).

Here are the top 5 Oncology Nursing News stories for August 2018.

The Food and Drug Administration (FDA) granted priority review to pembrolizumab (Keytruda) for the treatment of adults and children with recurrent locally advanced or metastatic Merkel cell carcinoma, a rare skin cancer.

The FDA launched a pilot program designed to advance innovative clinical trial designs.

As the cost of cancer therapies continues to increase, the cancer community is looking to biosimilars as a potential alternative for treatment; however, experts wonder if factors besides cost need to be taken in to consideration.

New guidelines issued by the US Preventive Services Task Force (USPSTF) recommend for women aged 30 to 65 years at average risk for cervical cancer to choose to receive a Pap smear alone every 3 years, screening with the high-risk human papillomavirus test alone, or cotesting every 5 years.

This year, the Foundation for Women’s Cancer (FWC) is collaborating with SHARE Cancer Support, National Ovarian Cancer Coalition (NOCC) and Tell Every Amazing Lady (T.E.A.L.®) on a national social media campaign featuring the voices of real people.

The Foundation for Women’s Cancer Continues the Fight to end gynecologic cancer one step at a time; race set for Nov. 4, 2018 in Washington, DC.

The Bonnie J. Addario Lung Cancer Foundation (ALCF) and the EGFR Resisters, a patient-driven community of people living with epidermal growth factor receptor (EGFR) positive lung cancer and their loved ones, are working together to raise funds and increase awareness of projects that benefit the EGFR community.
The trial that led to national screening guidelines—which recommend screening based on age and smoking history—did not include fair representation from all racial/ethnic groups.

A recent survey of physicians revealed that 17% of doctors with personal experience with cancer were more likely than those without to act against established guidelines to recommend that low-risk women receive ovarian cancer screening.

Broad-based genomic sequencing, while useful in identifying tumor mutations in patients with non-small cell lung cancer (NSCLC), may not improve survival outcomes when compared to routine genomic testing.

While patients may feel a sense of urgency in making a treatment decision, it’s important for patients to assess their own feelings about their goals for treatment.

Attempting to learn and process large amounts of complex information after a diagnosis of pancreatic cancer can leave anyone feeling lost. So where should you turn? Start with Patient Central.