
Proactive onco-coaching did not improve QOL but was associated with improved OS in patients with metastatic renal cell carcinoma receiving TKIs and ICIs.

Proactive onco-coaching did not improve QOL but was associated with improved OS in patients with metastatic renal cell carcinoma receiving TKIs and ICIs.
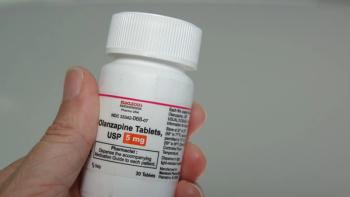
Patients treated with tyrosine kinase inhibitors received olanzapine to address adverse effects including nausea/vomiting, anorexia, insomnia, and weight loss.

Patients with recurrent/metastatic HER2-positive breast cancer experienced durable response and manageable safety from KN026-docetaxel combination therapy.

Lisocabtagene maraleucel demonstrated a statistically significant overall response rate in adults with relapsed/refractory marginal zone lymphoma.
New results uphold previous findings regarding the efficacy of cabozantinib, nivolumab, and ipilumumab arm for advanced renal cell carcinoma.
Patients with advanced RCC who were treated with nivolumab plus cabozantinib had a median PFS of 16.4 months compared with 8.3 months from sunitinib alone.
Men with intermediate-risk prostate cancer and high PSA levels before HIFU treatment had a greater risk of both overall recurrence and treatment failure.
Enfortumab vedotin alone and in combination with pembrolizumab showed promise in patients with upper tract urothelial carcinoma, particularly those ineligible for standard chemotherapy.

Real-world data demonstrate significant outcome improvements with enfortumab vedotin in patients with unresectable or metastatic urothelial carcinoma.

Patients with metastatic hormone-sensitive prostate cancer experienced increase rPFS and other efficacy end points with darolutamide plus ADT.

Improved progression-free survival in metastatic urothelial carcinoma was observed in patients experiencing neuropathy, skin rash, and hyperglycemia following enfortumab vedotin.

Increase in QOL and decrease in sexual function are among the benefits and risks, respectively, of adding SADT to EBRT for intermediate-risk prostate cancer.

Findings also suggested that an up-front dose reduction of enfortumab vedotin may benefit older patients with urothelial carcinoma.

MyCareGorithm was met with high approval from patients, companions, and physicians, with all 3 groups reporting they’d recommend the tool to other patients.

While lower relative dose intensity of docetaxel was associated with higher rates of G-CSF use and more adverse events, discontinuation rates were low across all groups.

Patients with mCRPC taking LuPSMA had a median OS of 34 months vs 26 months with enzalutamide alone.
With delays in biomarker testing and treatment for metastatic castration-resistant prostate cancer, continued education is needed to address these gaps.

Nurses and APPs provide an essential link between physicians and patients when facilitating and monitoring outcomes with personalized cancer vaccines.
Patients with HRR-deficient metastatic castration-resistant prostate cancer saw a 14-month boost in OS and a 38% lower risk of death with talazoparib plus enzalutamide.
Amezalpat has been granted FDA fast track designation as treatment for patients with hepatocellular carcinoma.

Findings from the ECHELON-3 trial supported the approval of brentuximab vedotin plus lenalidomide and rituximab for relapsed/refractory LBCL.

Results from the PEACOCC trial warrant further investigation into treatment with pembrolizumab in patients with previously treated CCGC.
Serial CTRS, an AI-powered tool, has been granted FDA breakthrough device designation for classifying patients with NSCLC.

Envafolimab plus suvemcitug and FOLFIRI showed early efficacy and manageable safety in MSS/pMMR colorectal cancer, according to phase 2 trial data.

Lerociclib plus fulvestrant demonstrated a progression-free survival (PFS) advantage across all patient subgroups with HR–positive, HER2–negative advanced breast cancer.

The FDA has granted breakthrough device designation to the ACR-368 OncoSignature assay for use in endometrial cancer.

Erica S. Doubleday, MS, FNP-C, BSN, RN, illustrated the importance of consistent care to identify AEs like interstitial lung disease in patients with cancer.
In a phase 1 trial, all 9 patients with renal cell carcinoma treated with a neoantigen-targeting personalized cancer vaccine showed no recurrence at data cutoff.
After a phase 3 study showed a promising pCR rate with HLX11, the pertuzumab biosimilar’s BLA has been accepted for review by the FDA.

More excellent responses occurred for patients with low-risk thyroid cancer who had thyroidectomies without radioiodine vs treatment with radioiodine.