KEYNOTE-B96 showed pembrolizumab-based therapy improved PFS and OS in PD-L1–positive platinum-resistant ovarian cancer.

KEYNOTE-B96 showed pembrolizumab-based therapy improved PFS and OS in PD-L1–positive platinum-resistant ovarian cancer.

A nurse practitioner gives her advice for managing adverse events during treatment with talquetamab for multiple myeloma.
CT0596 was well tolerated and showed preliminary efficacy in relapsed/refractory multiple myeloma, early phase 1 data show.
The combo showed safety and efficacy in patients with c-MET protein overexpression who progressed on prior osimertinib.
Neoadjuvant ribociclib plus endocrine therapy showed pCR rates comparable to chemo in HR-positive, HER2-negative early breast cancer.
Disitamab vedotin plus toripalimab improved PFS and OS over chemo in HER2+ metastatic urothelial cancer, per phase 3 RC48-C016 interim analysis.
Disitamab vedotin with toripalimab improved PFS and OS over chemo in HER2-expressing metastatic urothelial carcinoma, per phase 3 trial interim analysis.

The number of CDK4/6-targeting treatment options available for patients with HR-positive breast cancer allows providers to personalize treatment.
Three of 18 patients had remissions lasting over 2 years in a study of ibrutinib plus nivolumab for relapsed/refractory CNS lymphoma.

Retifanlimab has received approval for the frontline treatment of advanced anal cancer from the FDA.

The extended injection time for subcutaneous daratumumab in those with myeloma can serve as an opportunity for oncology nurses to check in with patients.
The addition of bevacizumab to capecitabine produced favorable efficacy and safety data for older patients with metastatic colorectal cancer.

Telisotuzumab vedotin-tllv has earned accelerated approval for use in patients with non-squamous non-small cell lung cancer with high c-Met overexpression.

Belzutifan has become the first FDA-approved oral therapy for pheochromocytoma or paraganglioma.
The phase 2b SURVIVE trial of SurVaxM in newly diagnosed glioblastoma will continue as planned following an interim review by an independent safety board.
T-DXd followed by THP improved pCR in high-risk, early-stage HER2+ breast cancer in the phase 3 DESTINY-Breast11 trial.
ADRX-0706 has received FDA fast track designation for the treatment of metastatic or locally advanced squamous cell cervical cancer.
Treatment-related adverse effects were reported in 97.2% of heavily pretreated patients with uterine serous carcinoma taking adavosertib.
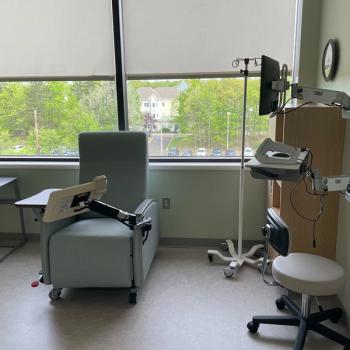
Infusion bays designed with nurse input provide patients the choice between privacy and community while allowing support from loved ones.
The safety and efficacy of givinostat is being assessed vs hydroxyurea in the phase 3 GIV-IN PV trial.
Use of the 1.5T Elekta Unity MR-Linac was associated with reduced rates of erectile dysfunction at 6, 12, and 18 months in patients with prostate cancer.

An update to the NCCN guidelines recommends naxitamab-gqgk for adult and pediatric patients with high-risk neuroblastoma.

The FDA has granted accelerated approval to avutometinib and defactinib in KRAS-mutated recurrent low-grade serous ovarian cancer.

Black and Hispanic pediatric patients with high-risk neuroblastoma had significantly lower 5-year survival rates in induction and/or consolidation trials.

Early trial data show 13.3% objective response with avutometinib, abemaciclib, and fulvestrant in HR+/HER2– breast cancer resistant to CDK4/6 inhibitors.

A registered nurse gives best practices for use of subcutaneous daratumumab in multiple myeloma.
The FDA granted a complete response letter with no safety concerns for the new drug application for TLX101-CDx’s use in glioma imaging.

New data show strong overall survival rates, building on previously reported outcomes with efti plus pembrolizumab for head and neck squamous cell carcinoma.
Early-phase LUPER trial results suggest the lurbinectedin/pembrolizumab combination may benefit patients with relapsed small cell lung cancer.
Adagrasib shows early signs of activity and tolerability in patients with STK11/KRAS G12C–mutated non–small cell lung cancer.