
A study published by researchers at the Dana Farber-Cancer Institute recently discovered that financial status plays a large role in the level of symptom burden and quality of life for patients newly diagnosed with lung or colorectal cancer.

A study published by researchers at the Dana Farber-Cancer Institute recently discovered that financial status plays a large role in the level of symptom burden and quality of life for patients newly diagnosed with lung or colorectal cancer.

Is there a treatment-related survival benefit for older patients with metastatic colorectal cancer?

Joseph Ravenell, MD, MS, from NYU Langone Medical Center, discusses access to screening among black men.

Racial disparities in mortality for breast cancer have gotten worse in women and persisted for colorectal cancer in men, although progress has been made in closing the mortality gap between blacks and whites for other cancers.

Joseph Ravenell, MD, MS, discusses some of the challenges with screening black men for colon cancer.

Danielle Novetsky Friedman, a general pediatrician in the Pediatric Long-Term Follow-Up Program at Memorial Sloan Kettering Cancer Center (MSK), discusses a study investigating the mechanisms leading to abnormal glucose and insulin dynamics in survivors of childhood cancers.

Robin B. Brenner, RN, CRN, OCN, clinical research nurse, David M. Rubinstein Center for Pancreatic Cancer Research, Memorial Sloan Kettering Cancer Center, discusses adverse events associated with Onivyde (irinotecan liposome injection; MM-398) for pancreatic cancer.

Results from clinical trials presented at the 2016 Gastrointestinal Cancers Symposium signal a role for the immunotherapy nivolumab (Opdivo) in the treatment of GI malignancies.

It is estimated that 1 million new cases are diagnosed in the world each year, and more than 723,000 deaths occur annually.

Michael Soulen, MD, a professor of radiology and surgery at the Abramson Cancer Center, University of Pennsylvania, discusses radioembolization to treat liver metastases in patients with neuroendocrine tumors (NETs) as well as the efficacy of embolization in treating NETs.

Entering through the giant, inflatable colon, dozens of patients, caregivers, family members and providers gathered at the Georgetown University Hotel and Conference Center in Washington, DC, December 5 for the symposium, “Fighting a Smarter War Against Cancer.â€

Disparities in colorectal cancer deaths have had a significant impact on the national economy, with areas of low socioeconomic status experiencing the greatest losses, according to a study by researchers at the Centers for Disease Control and Prevention (CDC).

Diagnosis and management guidelines.

Brian Untch, MD, assistant member, Department of Surgery, Gastric and Mixed Tumor Service, Head and Neck Service, Memorial Sloan Kettering Cancer Center, discusses symptoms of pancreatic neuroendocrine tumors (NETs).Â

The FDA has approved MM-398, branded as Onivyde (irinotecan liposome injection) in combination with 5-fluorouracil (5-FU) chemotherapy and leucovorin for patients with metastatic pancreatic cancer. The treatment follows prior administration of a gemcitabine-based regimen.

USPSTF issues draft recommendations.

The oral nucleoside TAS-102 (Lonsurf) received FDA approval today for the treatment of patients with advanced colorectal cancer (CRC) who are not responding to other treatments.
The US Preventive Services Task Force issued a draft guideline today recommending the use of low-dose aspirin for certain individuals for the prevention of CRC and cardiovascular disease.

For patients with stage III colon cancer, drinking four or more cups of coffee per day may reduce risk of recurrence by 42% and death by 34%.
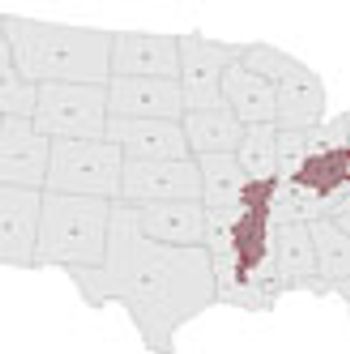
Researchers have identified three "hotspots" in the United States where progress on reducing death rates from colorectal cancer have lagged, shining a light on areas of the country where efforts to increase screening uptake and promote healthier lifestyles could make a big difference.

Though having a high body mass index is associated with a higher risk for colorectal cancer, patients who are thinner may not do as well after treatment for metastatic colorectal cancer.

Susan R. Mazanec, PhD, RN, AOCN, Research Assistant Professor, Frances Payne Bolton School of Nursing, Case Western Reserve University, discusses a study that examined activation for health management in colorectal cancer patients and their family caregivers during the transition to post-treatment survivorship.

Lynch syndrome (LS) is a hereditary syndrome that causes a marked increased risk of colorectal and other cancers. It is inherited in an autosomal dominant pattern, which means that it is due to a mutation in one copy of a gene (in LS, a DNA mismatch repair gene).
Oncology nurse navigators are known for identifying gaps in cancer care, being proactive in setting goals to address them, and designing specific interventions that lead not only to better-and measurable-outcomes but also to increased patient satisfaction.

A recent study joins a body of evidence suggesting that long-term, regular aspirin use is associated with a reduced risk for cancer, with the most dramatic reduction being seen in colorectal cancer incidence.