
Each month, we take a look back at the most popular Oncology Nursing News stories. Here are the top 5 stories from June 2020.

Each month, we take a look back at the most popular Oncology Nursing News stories. Here are the top 5 stories from June 2020.



Following the arrival of the coronavirus disease 2019 pandemic, St. Jude Children's Research Hospital launched the first Global COVID-19 Observatory and Resource Center for Childhood Cancer.

The FDA has approved pembrolizumab (Keytruda) for the first-line treatment of patients with unresectable or metastatic microsatellite instability–high or mismatch repair deficient colorectal cancer. This marks the first immunotherapy approved for this patient population as a first-line treatment and which is administered to patients without also giving chemotherapy.

The FDA approved subcutaneous Phesgo – a combination of pertuzumab (Perjeta), trastuzumab (Herceptin), and hyaluronidase–zzxf – for the treatment of patients with metastatic HER2-positive breast cancer, as well as early-stage HER2-positive breast cancer, as selected by an FDA-approved companion diagnostic test.
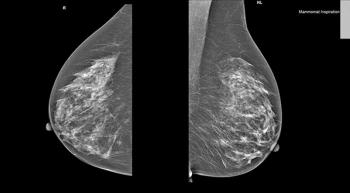
Patients with breast cancer who harbor multiple PIK3A-mutant tumors achieved a higher clinical benefit from PI3Kα inhibition compared with single mutant tumors according to response analysis data from the SANDPIPER trial.

The pandemic has reduced cancer screening and diagnostic testing as well as clinical trials and research.

Nivolumab plus ipilimumab showed an improvement in relapse-free survival for this patient population.

The FDA has approved pembrolizumab (Keytruda) or patients with recurrent or metastatic cutaneous squamous cell carcinoma that is not curable by surgery or radiation.

Pembrolizumab (Keytruda) demonstrated modest clinical activity in patients with advanced recurrent ovarian cancer, according to final results of the phase 2 KEYNOTE-100 trial that were presented during the 2020 ASCO Virtual Scientific Program.

The ongoing coronavirus disease 2019 (COVID-19) pandemic has magnified the value of real-world data (RWD) in oncology. The FDA is presently spearheading several initiatives aimed at refining the role of RWD in cancer care to guide clinical trial development, procure answers to pressing clinical questions, and support regulatory decisions for in vitro diagnostics.

The FDA has had a busy couple of weeks, approving new agents to treat a variety of cancers.

The combination of atezolizumab (Tecentriq) plus carboplatin/etoposide continued to demonstrate an improvement in overall survival (OS) versus chemotherapy alone as a frontline treatment for patients with extensive-stage small cell lung cancer (ES-SCLC), regardless of PD-L1 and blood tumor mutational burden (bTMB) status.

The FDA has approved selinexor as a treatment for adult patients with relapsed/refractory diffuse large B-cell lymphoma, not otherwise specified, who have received ≥2 prior therapies.

Significant differences were seen between Hispanic and non-Hispanic white patients with hematological malignancies in Texas in terms of the age of diagnosis and long-term survival outcomes.

The FDA approved tazemetostat to treat relapsed/refractory follicular lymphoma in 2 different indications.

The FDA has approved pembrolizumab (Keytruda) to treat adult and pediatric patients with unresectable or metastatic solid tumors that are tissue tumor mutational burden–high (≥10 mutations/megabase) and have progressed following prior therapy and who have no satisfactory alternative treatment options.

The FDA expanded the approval of gemtuzumab ozogamicin (Mylotarg) to include newly diagnosed pediatric patients (1 month or older) with CD33-positive acute myeloid leukemia (AML).

The FDA approved lurbinectedin (Zepzelca) for the treatment of adult patients with metastatic small cell lung cancer with disease progression, following platinum-based chemotherapy.

On Friday, the FDA expanded the approval for Gardasil 9 for the prevention of oropharyngeal and other head and neck cancers caused by human papillomavirus (HPV) types 16,18,31, 33, 45, 52, and 58, according to Merck, the manufacturer of the vaccine.
Ruxolitinib (Jakafi) induced a strong, durable response across several subgroups of patients with steroid-refractory acute graft-versus-host disease.

The FDA has approved pegfilgrastim-apgf (Nyvepria), a biosimilar to pegfilgrastim (Neulasta), to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia.
The 4-drug combination of atezolizumab (Tecentriq), bevacizumab (Avastin), carboplatin, and paclitaxel (ABCP) improved survival while maintaining good health-related quality of life (QOL) in patients with nonsquamous non–small cell lung cancer .


Telemedicine will remain a mainstay even after the COVID-19 pandemic, says Andrew M. Evens, DO, MSc.
Brad S. Kahl, MD, discusses the unique challenges that occur when treating patients with indolent non-Hodgkin lymphoma.

Efforts are being made to address remaining questions in the realm of BRAF-mutant melanoma, according to Charles L. Sawyers, MD.
Long-term data presented at the 2020 ASCO Virtual Scientific Program demonstrated that acalabrutinib (Calquence) is safe and effective in patients with treatment-naïve chronic lymphocytic leukemia, supporting its use in the front line setting in this population.

The FDA has granted margetuximab an Orphan Drug designation for the treatment of patients with gastric and gastroesophageal junction (GEJ) cancer, according to MacroGenics, Inc., the manufacturer of the Fc-engineered monoclonal antibody.