
Pembrolizumab represents the first immunotherapy approved to treat patients with renal cell carcinoma in the adjuvant setting.


Pembrolizumab represents the first immunotherapy approved to treat patients with renal cell carcinoma in the adjuvant setting.
The increased risk of prostate cancer associated with MSH2 and MSH6 pathogenic variants point to a need for annual PSA screening in men over the age of 40.
An expert from Dana-Farber Cancer Institute comments on ongoing research in translocation RCC and next steps for research in this treatment space.

The implementation of the Oncology Nursing Society’s new Get Up, Get Moving Program helped patients increase their daily step intake and maintain their health-related quality of life.
Approximately 73% of patients with NMIBC who experienced a complete response after non-surgical primary chemoablation using mitomycin-containing reverse thermal gel UGN-102 remained disease free at 9 months.
A real-world survival analysis showed that patients with metastatic RCC treated with axitinib plus pembrolizumab did not experience significantly improved survival outcomes compared with patients treated with ipilimumab plus nivolumab.
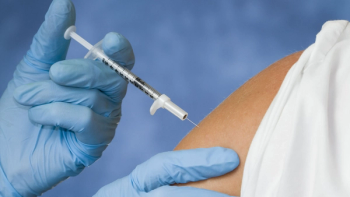
The Pfizer-BioNTech COVID-19 vaccine induces similar levels of COVID-19 antibodies in patients with solid cancer compared with people without cancer.

A recent study demonstrated that patients with prostate cancer on active surveillance reported reduced PSA levels after a 12-week HIIT regime.

Patients with prostate cancer fared better following treatment with magnetic resonance-guided focused ultrasound focal therapy than with radical prostatectomy or radiation therapy, shows study presented at 2021 AUA Annual Meeting.
Real-world evidence recently presented at the 2021 AUA Annual Meeting showed that high adherence rates and favorable prostate-specific antigen (PSA) response were observed in men with nmCRPC who were treated with apalutamide.
Patients with advanced RCC who received prior VEGF-targeted therapy tend to have longer survival outcomes with cabozantinib than with axitinib.

Obese men are more likely to have improved overall survival following treatment for mCRPC than man who are overweight or normal weight, analysis finds.

A global study revealed that patients and caregivers alike reported insufficient knowledge surrounding renal cell carcinoma.
Characteristics such as low positive PD-L1 expression and rare high positive staining were identified in both patients with VHL-mutated and VHL wild type clear cell renal cell carcinoma.
Patients with nonmetastatic castration-resistant prostate cancer often undergo prolonged treatment periods, making the safety profile of therapies like darolutamide an important factor in optimum treatment selection.

Yoga has a positive impact on the vagus nerve, which influences how people think, remember, and feel, and therefore can improve the mental well-being of patients with prostate cancer.
Researchers identified a slower rate of health-related quality of life deterioration, as well as an ability to postpone locally invasive procedures, in patients with nonmetastatic castration-resistant prostate cancer who received darolutamide.

Pembrolizumab has been granted full approval as a first-line treatment for a select population of patients with bladder cancer.

The Food and Drug Administration approved adjuvant nivolumab to treat urothelial carcinoma in patients who are at high risk of recurrence after undergoing radical resection, irrespective of prior neoadjuvant chemotherapy, nodal involvement or PD-L1 status.

Belzutifan, a hypoxia-inducible factor inhibitor, has been approved for treatment of adults with cancers related to von Hippel-Lindau disease.

Recently approved combination therapies have contributed to significantly improved survival rates in renal cell carcinoma.

The combination of pembrolizumab and lenvatinib has been FDA approved as a frontline therapy for the treatment of adult patients with advanced renal cell carcinoma.
Germline variation may cause men of African descent to have a higher risk of prostate than men of European descent.

Patients with metastatic castrate-resistant prostate cancer and PTEN-loss tumors experienced improved radiographical progression-free survival with ipatasertib plus abiraterone.
Patients with favorable-risk prostate cancer tended to move away from active surveillance to initiating treatment after receiving their Oncotype DX Genomic Prostate Score.