Lung Cancer
Latest News
Latest Videos

CME Content
More News

Fast track designation has been granted by the FDA to PT217 for extensive stage small cell lung cancer with disease progression.

Alectinib was approved for the treatment of patients with ALK-positive non-small cell lung cancer after surgical resection.
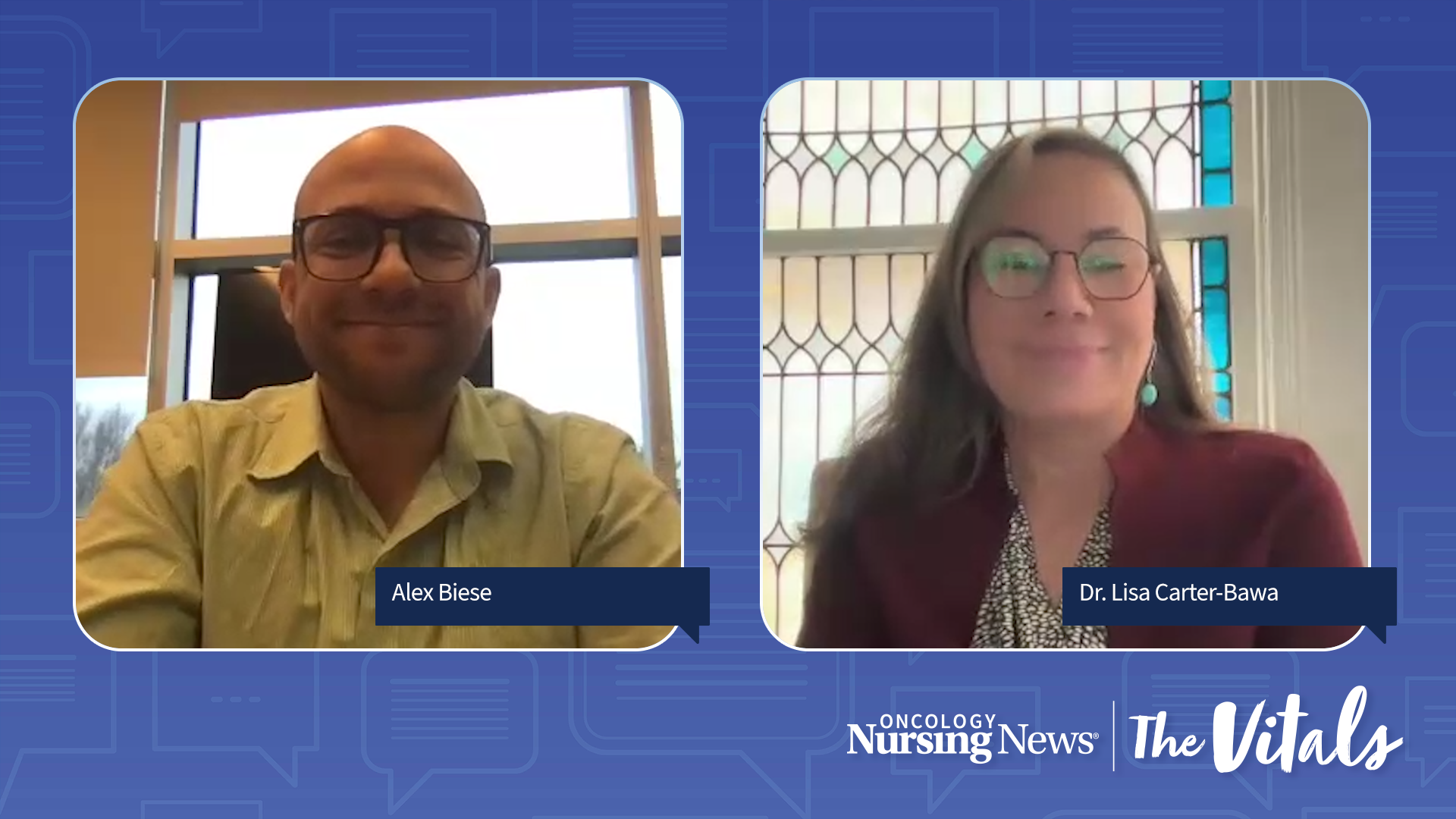
Oncology nurses play a vital role in informing patients about the importance of molecular testing and brain imaging in EGFR-mutated lung cancer.

Over the last 5 years, 57% of drugs approved by the FDA for a cancer-related indication did not show a clinical benefit in confirmatory studies.

Oncology nurses provide patients with lung cancer the education and empathy needed to navigate treatment options and adverse event management.

Compared with docetaxel, adagrasib improved progression-free survival and objective response rate in patients with pretreated KRAS G12C–mutant non–small cell lung cancer.
Tumor-treating fields significantly improved the time to intracranial progression in patients from the phase 3 METIS trial with brain metastases from non-small cell lung cancer.

First-line treatment with osimertinib and chemotherapy significantly improved survival outcomes after disease progression in EGFR-mutated NSCLC.

First-line treatment with anlotinib plus chemotherapy improved progression-free survival compared with chemotherapy plus placebo in patients with extensive-stage small cell lung cancer.

The preference of subcutaneous atezolizumab compared with its intravenous formulation was demonstrated in patients with non–small cell lung cancer treated on the IMscin002 trial.

Throughout March, the FDA has approved drugs for the treatment of disease including lung, hematologic, esophageal, and gynecologic cancers.

The benefits noted in this exploratory analysis were irrespective of the number of neoadjuvant treatment cycles completed in patients with resectable non-small cell lung cancer.

Patients with EGFR exon 20 insertion–mutated NSCLC treated with amivantamab plus chemotherapy experienced an extension in the time to treatment discontinuation and subsequent therapy.

Patients with non–small cell lung cancer treated with targeted therapies experienced an overall survival benefit.

Based on results from a post hoc analysis, researchers found that lurbinectedin was more effective and less toxic than topotecan for the treatment of small cell lung cancer.

Amivantamab was approved by the FDA as first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations or as therapy for those whose disease progressed on or after platinum-based chemotherapy.

Treatment with neoadjuvant tislelizumab plus platinum-based doublet chemotherapy, followed by surgery and adjuvant tislelizumab, improved event-free survival in patients with resectable non–small cell lung cancer.

The FDA is currently reviewing a BLA that seeks the approval of datopotamab deruxtecan as a therapeutic option for pretreated nonsquamous non–small cell lung cancer.

Lurbinectedin confers greater activity with a superior safety profile when compared with topotecan in a subset of patients with small cell lung cancer.
Oncology nurses can assist patients in understanding the significance of KRAS mutation testing and its treatment implications.

Stereotactic ablative radiotherapy with standard-of-care therapy improved progression-free survival in patients with oligoprogressive non-small cell lung cancer, although the benefit was not seen in those with oligoprogressive breast cancer.

The use of perioperative tislelizumab plus neoadjuvant chemotherapy has been supported by findings from the phase 3 RATIONALE-315 study as a treatment for resectable stage II to IIIA non–small cell lung cancer.

The FDA approved osimertinib for use with platinum-based chemotherapy to treat patients with locally advanced or metastatic non-small cell lung cancer that harbor EGFR exon 19 deletions or exon 21 L858R mutations.

Tepotinib has received a traditional approval from the FDA for metastatic NSCLC with MET exon 14 skipping alterations, updating the previous accelerated approval from 2021.
Dawn Landolph, RN, BSN, OCN, MPA, provides an in-depth look at adagrasib for the treatment of patients with KRAS G12C-mutated, locally advanced or metastatic non-small cell lung cancer in a downloadable reference sheet.