Lung Cancer
Latest News
Video Series

Latest Videos
Podcasts
CME Content
More News

Subcutaneous pembrolizumab has been approved across all indications, cutting chair and administration times with a median injection time of 2 minutes.
Zidesamtinib, a brain-penetrant, TRK-sparing ROS1 TKI, showed activity and manageable safety in pretreated patients with advanced ROS1+ NSCLC.
FLAURA2 data show osimertinib plus chemotherapy significantly improves overall survival vs osimertinib alone in frontline EGFR+ advanced NSCLC.
Lutetium Lu 177 dotatate was linked with partial responses in patients with metastatic bronchopulmonary neuroendocrine tumors, per real-world data.
Tepotinib was associated with frequent but manageable adverse events in MET exon 14–positive NSCLC, with peripheral edema most common.
Adding ivonescimab to chemotherapy improved PFS for patients with EGFR-mutated non–small cell lung cancer after treatment with a third-generation TKI.

Familiarity with each component of antibody-drug conjugates helps nurses and APPs deliver proactive adverse event management to patients with cancer.
Biology-guided radiotherapy involves teamwork across multiple specialists, according to Samantha Bianzon, BSN, RN.
The NCCN has added zongertinib to its guidelines for the care of patients with HER2 (ERBB2)-mutated non–small cell lung cancer.
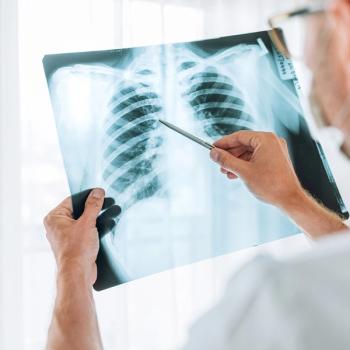
Findings from a small cohort of a phase 2b trial demonstrated 2-year OS benefit in patients with pulmonary metastatic osteosarcoma treated with OST-HER2.

FDA accelerated approval was given to zongertinib for the treatment of patients with unresectable/metastatic nonsquamous NSCLC with HER2 TKD activating mutations.
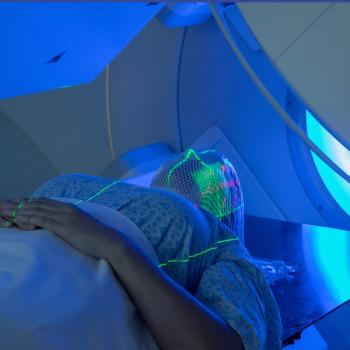
Nurses should be familiar with PET scan protocol and what to ask patients with lung and bone cancers before administering biology-guided radiation therapy.

New FDA approvals in July include therapies for NSCLC, relapsed multiple myeloma, liver cancer, and B-cell malignancies.
Oncology nurses can ensure patients with non–small cell lung cancer receive the optimal treatment quickly by understanding comprehensive genomic profiling.
2025 ICE-T Conference presenters explain what nurses and APPs should take into account as immune cell effector therapies become more widely used.
The investigational BET inhibitor is being investigated combined with abemaciclib or cisplatin/etoposide in NUT carcinoma in 2 clinical trials.
Most patients demonstrated at least partial response to tarlatamab in a real-world population with extensive-stage small cell lung cancer.
Panelists discuss how comprehensive patient education about expected adverse effects, combined with prophylactic measures including dexamethasone premedication and dermatologic support, helps patients successfully tolerate amivantamab plus lazertinib therapy while maintaining treatment adherence.

Panelists discuss how real-world experience with amivantamab plus lazertinib has shown remarkable responses consistent with trial data, including effectiveness in challenging cases such as leptomeningeal disease, with manageable adverse effects when proper prophylactic measures and patient education are implemented.

Sunvozertinib has received accelerated approval for use in advanced or metastatic non–small lung cancer harboring EGFR exon 20 insertion mutations.

Oncologic therapies approved in June included indications in genitourinary, lung, hematologic, and head and neck cancers.

Anlotinib combined with immune checkpoint inhibitors may benefit patients with extensive-stage small cell lung cancer treated with prior immunotherapy.

Panelists discuss how both amivantamab plus lazertinib and osimertinib-based regimens show good central nervous system (CNS) activity for patients with baseline brain metastases, with treatment choice influenced more by patient-specific factors such as bleeding risk and anticoagulation contraindications than by CNS efficacy differences.

Panelists discuss how the MARIPOSA study findings demonstrated significant progression-free survival and overall survival benefits with amivantamab plus lazertinib vs osimertinib monotherapy, with mature overall survival data showing a 25% improvement in survival outcomes.

Zirconium-89–labeled BMS-986279 demonstrated tumor uptake in fuc-GM1–positive lesions in patients with ES-SCLC.